HCV RNA decline in the first 24 h exhibits high negative predictive value of sustained virologic response in HIV/HCV genotype 1 co-infected patients treated with peginterferon and ribavirin
- PMID: 21376083
- PMCID: PMC3102437
- DOI: 10.1016/j.antiviral.2011.02.013
HCV RNA decline in the first 24 h exhibits high negative predictive value of sustained virologic response in HIV/HCV genotype 1 co-infected patients treated with peginterferon and ribavirin
Abstract
Background: Treatment with Peg-interferon and ribavirin (PEG-IFN/RBV) for HIV patients co-infected with hepatitis C virus (HCV) genotype 1 has suboptimal rates of response. Viral kinetics has emerged as one of the best prognostic factors of treatment outcome.
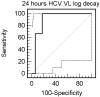
Methods: Twenty HIV/HCV genotype 1 co-infected patients in treatment with PEG-IFN/RBV, had blood drawn at baseline, 24 h, 4, 12, 24, 48, and 72 weeks. HCV-RNA levels were evaluated at each time point. ROC curves were used to evaluate the log10 HCV-RNA decay at 24 h that exhibits the best predictive value of achieving response. Genomic characterization of HCV NS5A at both interferon sensitivity-determining region (ISDR) and protein-kinase binding (PKRBD) domains were performed in order to evaluate its heterogeneity and association with 24 h HCV-RNA decay and SVR.
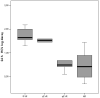
Results: Non-responder patients exhibited a mean of 0.7 log10 (SD 0.74 log10) HCV-RNA decay at 24 h, whereas responder-patients presented 1.6 log10 (SD 0.28 log10), p = 0.04. A reduction in HCV viral load from baseline to 24 h of < 1.4 had a negative predictive value for achieving SVR of 100% and a positive predictive value of 50%. HCV genotype 1 isolates from patients with a decrease of HCV-RNA at 24 h > 1.4 log10, exhibited 3.1(SD 1.5) amino acids substitutions in ISDR and 4.8(SD 2.3) in PKRBD regions and 1.6(SD 0.7) and 2.4(SD 1.3), respectively, in those patients presenting lower reduction in HCV-RNA.
Conclusions: HIV/HCV genotype 1 co-infected patients with a decrease in HCV-VL at 24 h > 1.4 log10 are more likely to achieve SVR when treated with PEG-IFN/RBV than those with lower levels of HCV-RNA decay. Along with other host-related and viral-related prognostic factors in HIV/HCV co-infected patients, this very early time point of evaluation could be of relevance in the management of HCV-specific treatment.
Copyright © 2011 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors do not have any commercial or other association that might pose a conflict of interest.
Figures


Similar articles
-
Early HCV dynamics on Peg-interferon and ribavirin in HIV/HCV co-infection: indications for the investigation of new treatment approaches.AIDS. 2004 Jan 2;18(1):59-66. doi: 10.1097/00002030-200401020-00007. AIDS. 2004. PMID: 15090830
-
Predictive value of early virologic response in HIV/hepatitis C virus-coinfected patients treated with an interferon-based regimen plus ribavirin.J Acquir Immune Defic Syndr. 2007 Feb 1;44(2):174-8. doi: 10.1097/QAI.0b013e31802b812d. J Acquir Immune Defic Syndr. 2007. PMID: 17106276 Clinical Trial.
-
Retreatment of hepatitis C patients with pegylated interferon combined with ribavirin in non-responders to interferon plus ribavirin. Is it different in real life?BMC Infect Dis. 2010 Jul 20;10:212. doi: 10.1186/1471-2334-10-212. BMC Infect Dis. 2010. PMID: 20646277 Free PMC article.
-
HCV viral decline at week 2 of Peg-IFN-alpha-2a/RBV therapy as a predictive tool for tailoring treatment in HIV/HCV genotype 1 co-infected patients.PLoS One. 2014 Jun 19;9(6):e99468. doi: 10.1371/journal.pone.0099468. eCollection 2014. PLoS One. 2014. PMID: 24945348 Free PMC article.
-
Development of novel therapies for hepatitis C.Antiviral Res. 2010 Apr;86(1):79-92. doi: 10.1016/j.antiviral.2010.02.003. Antiviral Res. 2010. PMID: 20417376 Review.
Cited by
-
Treatment outcomes of treatment-naïve Hepatitis C patients co-infected with HIV: a systematic review and meta-analysis of observational cohorts.PLoS One. 2013;8(2):e55373. doi: 10.1371/journal.pone.0055373. Epub 2013 Feb 5. PLoS One. 2013. PMID: 23393570 Free PMC article.
-
Chronic hepatitis C treatment outcomes in low- and middle-income countries: a systematic review and meta-analysis.Bull World Health Organ. 2012 Jul 1;90(7):540-50. doi: 10.2471/BLT.11.097147. Epub 2012 Feb 3. Bull World Health Organ. 2012. PMID: 22807600 Free PMC article.
References
-
- Amorosa VK, Slim J, Mounzer K, Bruno C, Hoffman-Terry M, Dorey-Stein Z, Ferrara T, Kostman JR, Lo Re V., 3rd The influence of abacavir and other antiretroviral agents on virological response to HCV therapy among antiretroviral-treated HIV-infected patients. Antiviral Ther. 2010;15:91–99. - PMC - PubMed
-
- Arends JE, Stuart JC, Baak LC, van der Ende ME, van Erpecum KJ, Simons CP, Boland GJ, van Baarle D, Hoepelman AI. Plasma HCV-RNA decline in the first 48 h identifies hepatitis C virus mono-infected but not HCV/HIV-coinfected patients with an undetectable HCV viral load at week 4 of peginterferon-alfa-2a/ribavirin therapy. J Viral Hepat. 2009;16:867–875. - PubMed
-
- Bolcic F, Bull L, Martinez L, Reynoso R, Salomon H, Arduino R, Barnett B, Quarleri J. Analysis of sequence configurations of the PKR-interacting HCV proteins from plasma and PBMC as predictors of response to interferon-alpha and ribavirin therapy in HIV-coinfected patients. Intervirology. 2008;51:261–264. - PubMed
-
- Bortoletto G, Scribano L, Realdon S, Marcolongo M, Mirandola S, Franceschini L, Bonisegna S, Noventa F, Plebani M, Martines D, Alberti A. Hyperinsulinaemia reduces the 24-h virological response to PEG-interferon therapy in patients with chronic hepatitis C and insulin resistance. J Viral Hepat. 2010;17:475–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

