TLR signaling is required for Salmonella typhimurium virulence
- PMID: 21376231
- PMCID: PMC3063366
- DOI: 10.1016/j.cell.2011.01.031
TLR signaling is required for Salmonella typhimurium virulence
Abstract
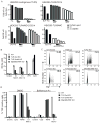
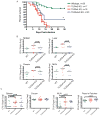
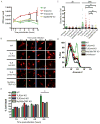
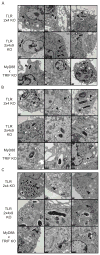
Toll-like receptors (TLRs) contribute to host resistance to microbial pathogens and can drive the evolution of virulence mechanisms. We have examined the relationship between host resistance and pathogen virulence using mice with a functional allele of the nramp-1 gene and lacking combinations of TLRs. Mice deficient in both TLR2 and TLR4 were highly susceptible to the intracellular bacterial pathogen Salmonella typhimurium, consistent with reduced innate immune function. However, mice lacking additional TLRs involved in S. typhimurium recognition were less susceptible to infection. In these TLR-deficient cells, bacteria failed to upregulate Salmonella pathogenicity island 2 (SPI-2) genes and did not form a replicative compartment. We demonstrate that TLR signaling enhances the rate of acidification of the Salmonella-containing phagosome, and inhibition of this acidification prevents SPI-2 induction. Our results indicate that S. typhimurium requires cues from the innate immune system to regulate virulence genes necessary for intracellular survival, growth, and systemic infection.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Host defenses trigger salmonella's arsenal.Cell Host Microbe. 2011 Mar 17;9(3):167-168. doi: 10.1016/j.chom.2011.03.003. Cell Host Microbe. 2011. PMID: 21402352
-
Bacterial Virulence: With a little help from my enemies.Nat Rev Microbiol. 2011 May;9(5):315. doi: 10.1038/nrmicro2561. Epub 2011 Mar 28. Nat Rev Microbiol. 2011. PMID: 21494276 No abstract available.
References
-
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. - PubMed
-
- Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. - PubMed
-
- Bellamy R, Ruwende C, Corrah T, McAdam KP, Whittle HC, Hill AV. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N Engl J Med. 1998;338:640–644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

