Astrocyte-neuron lactate transport is required for long-term memory formation
- PMID: 21376239
- PMCID: PMC3073831
- DOI: 10.1016/j.cell.2011.02.018
Astrocyte-neuron lactate transport is required for long-term memory formation
Abstract
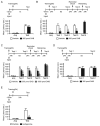
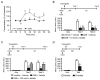
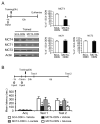
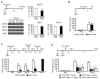
We report that, in the rat hippocampus, learning leads to a significant increase in extracellular lactate levels that derive from glycogen, an energy reserve selectively localized in astrocytes. Astrocytic glycogen breakdown and lactate release are essential for long-term but not short-term memory formation, and for the maintenance of long-term potentiation (LTP) of synaptic strength elicited in vivo. Disrupting the expression of the astrocytic lactate transporters monocarboxylate transporter 4 (MCT4) or MCT1 causes amnesia, which, like LTP impairment, is rescued by L-lactate but not equicaloric glucose. Disrupting the expression of the neuronal lactate transporter MCT2 also leads to amnesia that is unaffected by either L-lactate or glucose, suggesting that lactate import into neurons is necessary for long-term memory. Glycogenolysis and astrocytic lactate transporters are also critical for the induction of molecular changes required for memory formation, including the induction of phospho-CREB, Arc, and phospho-cofilin. We conclude that astrocyte-neuron lactate transport is required for long-term memory formation.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Astrocytes: powering memory.Cell. 2011 Mar 4;144(5):644-5. doi: 10.1016/j.cell.2011.02.027. Cell. 2011. PMID: 21376229
References
-
- Bamburg JR, McGough A, Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell. Biol. 1999;9:364–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

