Leptin enhances NMDA-induced spinal excitation in rats: A functional link between adipocytokine and neuropathic pain
- PMID: 21376468
- PMCID: PMC3098907
- DOI: 10.1016/j.pain.2011.01.054
Leptin enhances NMDA-induced spinal excitation in rats: A functional link between adipocytokine and neuropathic pain
Abstract
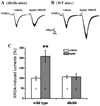
Recent studies have shown that leptin (an adipocytokine) played an important role in nociceptive behavior induced by nerve injury, but the cellular mechanism of this action remains unclear. Using the whole-cell patch-clamp recording from rat's spinal cord slices, we showed that superfusion of leptin onto spinal cord slices dose-dependently enhanced N-methyl-d-aspartate (NMDA) receptor-mediated currents in spinal cord lamina II neurons. At the cellular level, the effect of leptin on spinal NMDA-induced currents was mediated through the leptin receptor and the JAK2/STAT3 (but not PI3K or MAPK) pathway, as the leptin effect was abolished in leptin receptor-deficient (db/db) mice and inhibited by a JAK/STAT inhibitor. Moreover, we demonstrated in naïve rats that a single intrathecal administration of leptin enhanced spontaneous biting, scratching, and licking behavior induced by intrathecal NMDA and that repeated intrathecal administration of leptin elicited thermal hyperalgesia and mechanical allodynia, which was attenuated by the noncompetitive NMDA receptor antagonist MK-801. Intrathecal leptin also upregulated the expression of NMDA receptors and pSTAT3 within the rat's spinal cord dorsal horn, and intrathecal MK-801 attenuated this leptin effect as well. Our data demonstrate a relationship between leptin and NMDA receptor-mediated spinal neuronal excitation and its functional role in nociceptive behavior. Since leptin contributes to nociceptive behavior induced by nerve injury, the present findings suggest an important cellular link between the leptin's spinal effect and the NMDA receptor-mediated cellular mechanism of neuropathic pain. A functional link is demonstrated between leptin, an adipocytokine, and the cellular mechanisms of neuropathic pain via enhancement of function and expression of spinal N-methyl-d-aspartate receptors.
Copyright © 2011 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ahima RS, Qi Y, Singhal NS, Jackson MB, Scherer PE. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55:S145–S154. - PubMed
-
- Banks WA. The many lives of leptin. Peptides. 2004;25:331–338. - PubMed
-
- Bjørbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res. 2004;59:305–331. - PubMed
-
- Bjorkman R, Hallman KM, Hedner J, Hedner T, Henning M. Acetaminophen blocks spinal hyperalgesia induced by NMDA and substance P. Pain. 1994;57:259–264. - PubMed
-
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

