Transport of germ plasm on astral microtubules directs germ cell development in Drosophila
- PMID: 21376599
- PMCID: PMC3062663
- DOI: 10.1016/j.cub.2011.01.073
Transport of germ plasm on astral microtubules directs germ cell development in Drosophila
Abstract
Background: In many organisms, germ cells are segregated from the soma through the inheritance of the specialized germ plasm, which contains mRNAs and proteins that specify germ cell fate and promote germline development. Whereas germ plasm assembly has been well characterized, mechanisms mediating germ plasm inheritance are poorly understood. In the Drosophila embryo, germ plasm is anchored to the posterior cortex, and nuclei that migrate into this region give rise to the germ cell progenitors, or pole cells. How the germ plasm interacts with these nuclei for pole cell induction and is selectively incorporated into the forming pole cells is not known.
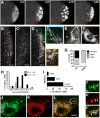
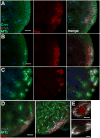
Results: Live imaging of two conserved germ plasm components, nanos mRNA and Vasa protein, revealed that germ plasm segregation is a dynamic process involving active transport of germ plasm RNA-protein complexes coordinated with nuclear migration. We show that centrosomes accompanying posterior nuclei induce release of germ plasm from the cortex and recruit these components by dynein-dependent transport on centrosome-nucleated microtubules. As nuclei divide, continued transport on astral microtubules partitions germ plasm to daughter nuclei, leading to its segregation into pole cells. Disruption of these transport events prevents incorporation of germ plasm into pole cells and impairs germ cell development.
Conclusions: Our results indicate that active transport of germ plasm is essential for its inheritance and ensures the production of a discrete population of germ cell progenitors endowed with requisite factors for germline development. Transport on astral microtubules may provide a general mechanism for the segregation of cell fate determinants.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures




References
-
- Strome S, Lehmann R. Germ versus soma decisions: lessons from flies and worms. Science. 2007;316:392–393. - PubMed
-
- Mahowald AP. Assembly of the Drosophila germ plasm. Int Rev Cytol. 2001;203:187–213. - PubMed
-
- Foe VE, Alberts BM. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 1983;61:31–70. - PubMed
-
- Santos AC, Lehmann R. Germ cell specification and migration in Drosophila and beyond. Curr Biol. 2004;14:R578–589. - PubMed
-
- Gavis ER, Lehmann R. Localization of nanos RNA controls embryonic polarity. Cell. 1992;71:301–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

