Absence of polo-like kinase 3 in mice stabilizes Cdc25A after DNA damage but is not sufficient to produce tumors
- PMID: 21376736
- PMCID: PMC7364384
- DOI: 10.1016/j.mrfmmm.2011.02.006
Absence of polo-like kinase 3 in mice stabilizes Cdc25A after DNA damage but is not sufficient to produce tumors
Abstract
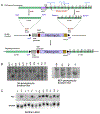
The polo-like kinases (Plks1-5) are emerging as an important class of proteins involved in many facets of cell cycle regulation and response to DNA damage and stress. Here we show that Plk3 phosphorylates the key cell cycle protein phosphatase Cdc25A on two serine residues in its cyclinB/cdk1 docking domain and regulates its stability in response to DNA damage. We generated a Plk3 knock-out mouse and show that Cdc25A protein from Plk3-deficient cells is less susceptible to DNA damage-mediated degradation than cells with functional Plk3. We also show that absence of Plk3 correlates with loss of the G1/S cell cycle checkpoint. However, neither this compromised DNA damage checkpoint nor reduced susceptibility to proteasome-mediated degradation after DNA damage translated into a significant increase in tumor incidence in the Plk3-deficient mice.
Copyright © 2011 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
None.
Figures
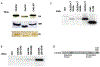





References
-
- Glover DM, Hagan IM, Tavares AAM, Polo-like kinases: a team that plays throughout mitosis, Genes Dev. 12 (1998) 3777–3787. - PubMed
-
- Barr FA, Sillje HH, Nigg EA, Polo-like kinases and the orchestration of cell division, Nat. Rev. Mol. Cell Biol 5 (2004) 429–440. - PubMed
-
- Moutinho-Santos T, Sampaio P, Amorim I, Costa M, Sunkel CE, In vivo localisation of the mitotic POLO kinase shows a highly dynamic association with the mitotic apparatus during early embryogenesis in Drosophila, Biol. Cell 91 (1999) 585–596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

