Functional connectivity MRI in infants: exploration of the functional organization of the developing brain
- PMID: 21376813
- PMCID: PMC3089442
- DOI: 10.1016/j.neuroimage.2011.02.073
Functional connectivity MRI in infants: exploration of the functional organization of the developing brain
Abstract
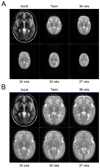
Advanced neuroimaging techniques have been increasingly applied to the study of preterm and term infants in an effort to further define the functional cerebral architecture of the developing brain. Despite improved understanding of the complex relationship between structure and function obtained through these investigations, significant questions remain regarding the nature, location, and timing of the maturational changes which occur during early development. Functional connectivity magnetic resonance imaging (fcMRI) utilizes spontaneous, low frequency (< 0.1 Hz), coherent fluctuations in blood oxygen level dependent (BOLD) signal to identify networks of functional cerebral connections. Due to the intrinsic characteristics of its image acquisition and analysis, fcMRI offers a novel neuroimaging approach well suited to investigation of infants. Recently, this methodology has been successfully applied to examine neonatal populations, defining normative patterns of large-scale neural network development in the maturing brain. The resting-state networks (RSNs) identified in these studies reflect the evolving cerebral structural architecture, presumably driven by varied genetic and environmental influences. Principal features of these investigations and their role in characterization of the tenets of neural network development during this critical developmental period are highlighted in this review. Despite these successes, optimal methods for fcMRI data acquisition and analysis for this population have not yet been defined. Further, appropriate schemes for interpretation and translation of fcMRI results remain unknown, a matter of increasing importance as functional neuroimaging findings are progressively applied in the clinical arena. Notwithstanding these concerns, fcMRI provides insight into the earliest forms of cerebral connectivity and therefore holds great promise for future neurodevelopmental investigations.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing financial interests or conflicts of interest.
Figures
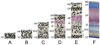
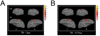


References
-
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

