Ascl3 knockout and cell ablation models reveal complexity of salivary gland maintenance and regeneration
- PMID: 21377457
- PMCID: PMC3093111
- DOI: 10.1016/j.ydbio.2011.02.025
Ascl3 knockout and cell ablation models reveal complexity of salivary gland maintenance and regeneration
Abstract
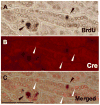
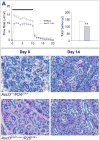
Expression of the transcription factor, Ascl3, marks a population of adult progenitor cells, which can give rise to both acinar and duct cell types in the murine salivary glands. Using a previously reported Ascl3(EGFP-Cre/+) knock-in strain, we demonstrate that Ascl3-expressing cells represent a molecularly distinct, and proliferating population of progenitor cells located in salivary gland ducts. To investigate both the role of the Ascl3 transcription factor, and the role of the cells in which it is expressed, we generated knockout and cell-specific ablation models. Ascl3 knockout mice develop smaller salivary glands than wild type littermates, but secrete saliva normally. They display a lower level of cell proliferation, consistent with their smaller size. In the absence of Ascl3, the cells maintain their progenitor function and continue to generate both acinar and duct cells. To directly test the role of the progenitor cells, themselves, in salivary gland development and regeneration, we used Cre-activated expression of diphtheria toxin (DTA) in the Ascl3-expressing (Ascl3+) cell population, resulting in specific cell ablation of Ascl3+ cells. In the absence of the Ascl3+ progenitor cells, the mice developed morphologically normal, albeit smaller, salivary glands able to secrete saliva. Furthermore, in a ductal ligation model of salivary gland injury, the glands of these mice were able to regenerate acinar cells. Our results indicate that Ascl3+ cells are active proliferating progenitors, but they are not the only precursors for salivary gland development or regeneration. We conclude that maintenance of tissue homeostasis in the salivary gland must involve more than one progenitor cell population.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–93. - PubMed
-
- Bekhor I, Wen Y, Shi S, Hsieh CH, Denny PA, Denny PC. cDNA cloning, sequencing and in situ localization of a transcript specific to both sublingual demilune cells and parotid intercalated duct cells in mouse salivary glands. Arch Oral Biol. 1994;39:1011–22. - PubMed
-
- Chang WW. Cell population changes during acinus formation in the postnatal rat submandibular gland. Anat Rec. 1974;178:187–201. - PubMed
-
- Dardick I, Burford-Mason AP. Current status of histogenetic and morphogenetic concepts of salivary gland tumorigenesis. Crit Rev Oral Biol Med. 1993;4:639–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

