Norepinephrine and ß₁-adrenergic signaling facilitate activation of hippocampal CA1 pyramidal neurons during contextual memory retrieval
- PMID: 21377513
- PMCID: PMC3074031
- DOI: 10.1016/j.neuroscience.2011.02.049
Norepinephrine and ß₁-adrenergic signaling facilitate activation of hippocampal CA1 pyramidal neurons during contextual memory retrieval
Abstract
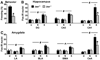
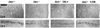
We previously described a role for adrenergic signaling in the hippocampus to promote contextual and spatial memory retrieval. A subsequent study performing expression analysis of the immediate-early gene (IEG) Arc suggested that activation of CA1 but not CA3 pyramidal neurons during memory retrieval is impaired in the absence of NE. The current study sought to confirm and extend those observations by performing expression analysis of a second IEG product, Fos, following a much greater variety of testing conditions. In mutant mice lacking NE, induction of Fos was normal in all regions of the hippocampus and amygdala shortly after fear conditioning. In contrast, when testing contextual fear 1 day after training, induction of Fos in CA1 and the central nucleus of the amygdala (CeA), but not CA3, the dentate gyrus or other amygdaloid nuclei, was impaired in the mutant mice. This pattern corresponded to the memory retrieval deficit exhibited by these mice. On the other hand, induction was normal in CA1 and CeA when testing cued fear 1 day after training, or contextual fear 1 week or 1 month after training, conditions in which retrieval are normal in the absence of NE. Acute restoration of NE in the mutant mice before testing but not before training rescued retrieval of contextual fear and restored Fos induction in CA1 and CeA. Because NE facilitates retrieval through the activation of β(1)-adrenergic receptors, β(1) knockout mice were also examined and found to exhibit reduced induction of Fos in CA1 and CeA following retrieval. Based on these and previous results, we hypothesize that adrenergic signaling is critical for the full activation of CA1 pyramidal neurons in response to excitatory input from CA3 pyramidal neurons conveying retrieved contextual information.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures






References
-
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. - PubMed
-
- Booze RM, Crisostomo EA, Davis JN. Beta-adrenergic receptors in the hippocampal and retrohippocampal regions of rats and guinea pigs: autoradiographic and immunohistochemical studies. Synapse. 1993;13:206–214. - PubMed
-
- Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–1215. - PubMed
-
- Clayton DF. The genomic action potential. Neurobiol Learn Mem. 2000;74:185–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

