Combined treatment of Pseudomonas aeruginosa biofilm with lactoferrin and xylitol inhibits the ability of bacteria to respond to damage resulting from lactoferrin iron chelation
- PMID: 21377840
- PMCID: PMC7008007
- DOI: 10.1016/j.ijantimicag.2010.12.019
Combined treatment of Pseudomonas aeruginosa biofilm with lactoferrin and xylitol inhibits the ability of bacteria to respond to damage resulting from lactoferrin iron chelation
Abstract
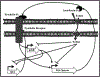
With an ageing and ever more obese population, chronic wounds such as diabetic ulcers, pressure ulcers and venous leg ulcers are an increasingly relevant medical concern. Identification of bacterial biofilm contamination as a major contributor to non-healing wounds demands biofilm-targeted strategies to manage chronic wounds. Pseudomonas aeruginosa has been identified as a principal biofilm-forming opportunistic pathogen in chronic wounds. The innate immune molecule lactoferrin and the rare sugar alcohol xylitol have been demonstrated to be co-operatively efficacious against P. aeruginosa biofilms in vitro. Data presented here propose a model for the molecular mechanism behind this co-operative antimicrobial effect. Lactoferrin iron chelation was identified as the primary means by which lactoferrin destabilises the bacterial membrane. By microarray analysis, 183 differentially expressed genes of ≥ 1.5-fold difference were detected. Interestingly, differentially expressed transcripts included the operon encoding components of the pyochelin biosynthesis pathway. Furthermore, siderophore detection verified that xylitol is the component of this novel synergistic treatment that inhibits the ability of the bacteria to produce siderophores under conditions of iron restriction. The findings presented here demonstrate that whilst lactoferrin treatment of P. aeruginosa biofilms results in destabilisation of the bacterial cell membrane though iron chelation, combined treatment with lactoferrin and xylitol inhibits the ability of P. aeruginosa biofilms to respond to environmental iron restriction.
Copyright © 2011 Elsevier B.V. and the International Society of Chemotherapy. All rights reserved.
Figures
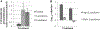



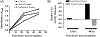
References
-
- James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. - PubMed
-
- Konturek PC, Brzozowski T, Konturek SJ, Kwiecien S, Dembinski A, Hahn EG. Influence of bacterial lipopolysaccharide on healing of chronic experimental ulcer in rat. Scand J Gastroenterol 2001;36:1239–47. - PubMed
-
- Power C, Wang JH, Sookhai S, Street JT, Redmond HP. Bacterial wall products induce downregulation of vascular endothelial growth factor receptors on endothelial cells via a CD14-dependent mechanism: implications for surgical wound healing. J Surg Res 2001;101:138–45. - PubMed
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318–22. - PubMed
-
- Rhoads DD, Wolcott RD, Percival SL. Biofilms in wounds: management strategies. J Wound Care 2008;17:502–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

