Community structures of fecal bacteria in cattle from different animal feeding operations
- PMID: 21378055
- PMCID: PMC3126396
- DOI: 10.1128/AEM.02988-10
Community structures of fecal bacteria in cattle from different animal feeding operations
Abstract
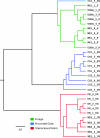
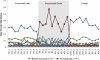
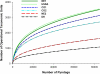
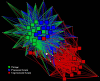
The fecal microbiome of cattle plays a critical role not only in animal health and productivity but also in food safety, pathogen shedding, and the performance of fecal pollution detection methods. Unfortunately, most published molecular surveys fail to provide adequate detail about variability in the community structures of fecal bacteria within and across cattle populations. Using massively parallel pyrosequencing of a hypervariable region of the rRNA coding region, we profiled the fecal microbial communities of cattle from six different feeding operations where cattle were subjected to consistent management practices for a minimum of 90 days. We obtained a total of 633,877 high-quality sequences from the fecal samples of 30 adult beef cattle (5 individuals per operation). Sequence-based clustering and taxonomic analyses indicate less variability within a population than between populations. Overall, bacterial community composition correlated significantly with fecal starch concentrations, largely reflected in changes in the Bacteroidetes, Proteobacteria, and Firmicutes populations. In addition, network analysis demonstrated that annotated sequences clustered by management practice and fecal starch concentration, suggesting that the structures of bovine fecal bacterial communities can be dramatically different in different animal feeding operations, even at the phylum and family taxonomic levels, and that the feeding operation is a more important determinant of the cattle microbiome than is the geographic location of the feedlot.
Figures


References
-
- Archibeque S. L., Miller D. N., Freetly H. C., Ferrell C. L. 2006. Feeding high-moisture corn instead of dry-rolled corn reduces odorous compound production in manure of finishing beef cattle with decreasing performance. J. Anim. Sci. 84:1767–1777 - PubMed
-
- Armstrong G. L., Hollingsworth J., Morris J. G. 1996. Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiol. Rev. 18:29–51 - PubMed
-
- Benenson A. S. 1995. Control of communicable diseases manual, 16th ed. American Public Health Association, Washington, DC
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

