Characterization of mutants deficient in the L,D-carboxypeptidase (DacB) and WalRK (VicRK) regulon, involved in peptidoglycan maturation of Streptococcus pneumoniae serotype 2 strain D39
- PMID: 21378199
- PMCID: PMC3133071
- DOI: 10.1128/JB.01555-10
Characterization of mutants deficient in the L,D-carboxypeptidase (DacB) and WalRK (VicRK) regulon, involved in peptidoglycan maturation of Streptococcus pneumoniae serotype 2 strain D39
Abstract
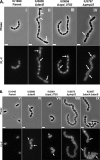
Peptidoglycan (PG) hydrolases play critical roles in the remodeling of bacterial cell walls during division. PG hydrolases have been studied extensively in several bacillus species, such as Escherichia coli and Bacillus subtilis, but remain relatively uncharacterized in ovococcus species, such as Streptococcus pneumoniae (pneumococcus). In this work, we identified genes that encode proteins with putative PG hydrolytic domains in the genome of S. pneumoniae strain D39. Knockout mutations in these genes were constructed, and the resulting mutants were characterized in comparison with the parent strain for growth, cell morphology, PG peptide incorporation, and in some cases, PG peptide composition. In addition, we characterized deletion mutations in nonessential genes of unknown function in the WalRK(Spn) two-component system regulon, which also contains the essential pcsB cell division gene. Several mutants did not show overt phenotypes, which is perhaps indicative of redundancy. In contrast, two new mutants showed distinct defects in PG biosynthesis. One mutation was in a gene designated dacB (spd_0549), which we showed encodes an L,D-carboxypeptidase involved in PG maturation. Notably, dacB mutants, similar to dacA (D,D-carboxypeptidase) mutants, exhibited defects in cell shape and septation, consistent with the idea that the availability of PG peptide precursors is important for proper PG biosynthesis. Epistasis analysis indicated that DacA functions before DacB in D-Ala removal, and immunofluorescence microscopy showed that DacA and DacB are located over the entire surface of pneumococcal cells. The other mutation was in WalRK(Spn) regulon gene spd_0703, which encodes a putative membrane protein that may function as a type of conserved streptococcal shape, elongation, division, and sporulation (SEDS) protein.
Figures



References
-
- Bateman A., Rawlings N. D. 2003. The CHAP domain: a large family of amidases including GSP amidase and peptidoglycan hydrolases. Trends Biochem. Sci. 28:234–237 - PubMed
-
- Buist G., Steen A., Kok J., Kuipers O. P. 2008. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol. Microbiol. 68:838–847 - PubMed
-
- Cabre M. 2009. Pneumonia in the elderly. Curr. Opin. Pulm. Med. 15:223–229 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

