Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells
- PMID: 21378261
- PMCID: PMC3208618
- DOI: 10.1165/rcmb.2010-0446OC
Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells
Abstract
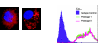
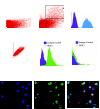
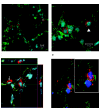
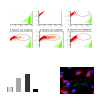
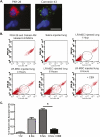
Multipotent mesenchymal progenitor cells, termed "mesenchymal stem cells" (MSCs), have been demonstrated to reside in human adult lungs. However, there is little information regarding the associations of these local mesenchymal progenitors with other resident somatic cells and their potential for therapeutic use. Here we provide in vivo and in vitro evidence for the ability of human adult lung-resident MSCs (LR-MSCs) to interact with the local epithelial cells. The in vivo retention and localization of human LR-MSCs in an alveolar microenvironment was investigated by placing PKH-26 or DsRed lentivirus-labeled human LR-MSCs in the lungs of immunodeficient (SCID) mice. At 3 weeks after intratracheal administration, 19.3 ± 3.21% of LR-MSCs were recovered, compared with 3.47 ± 0.51% of control fibroblasts, as determined by flow cytometry. LR-MSCs were found to persist in murine lungs for up to 6 months and demonstrated preferential localization to the corners of the alveoli in close proximity to type II alveolar epithelial cells, the progenitor cells of the alveolar epithelium. In vitro, LR-MSCs established gap junction communications with lung alveolar and bronchial epithelial cells and demonstrated an ability to secrete keratinocyte growth factor, an important modulator of epithelial cell proliferation and differentiation. Gap junction communications were also demonstrable between LR-MSCs and resident murine cells in vivo. This study demonstrates, for the first time, an ability of tissue-specific MSCs to engraft in their organ of origin and establishes a pathway of bidirectional interaction between these mesenchymal progenitors and adult somatic epithelial cells in the lung.
Figures


References
-
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 1970;3:393–403 - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147 - PubMed
-
- Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev 2006;20:161–171 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

