Total synthesis and study of 6-deoxyerythronolide B by late-stage C-H oxidation
- PMID: 21378935
- PMCID: PMC3274347
- DOI: 10.1038/nchem.351
Total synthesis and study of 6-deoxyerythronolide B by late-stage C-H oxidation
Abstract
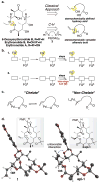
Among the frontier challenges in chemistry in the twenty-first century are the interconnected goals of increasing synthetic efficiency and diversity in the construction of complex molecules. Oxidation reactions of C-H bonds, particularly when applied at late stages of complex molecule syntheses, hold special promise for achieving both these goals. Here we report a late-stage C-H oxidation strategy in the total synthesis of 6-deoxyerythronolide B (6-dEB), the aglycone precursor to the erythromycin antibiotics. An advanced intermediate is cyclized to give the 14-membered macrocyclic core of 6-dEB using a late-stage (step 19 of 22) C-H oxidative macrolactonization reaction that proceeds with high regio-, chemo- and diastereoselectivity (>40:1). A chelate-controlled model for macrolactonization predicted the stereochemical outcome of C-O bond formation and guided the discovery of conditions for synthesizing the first diastereomeric 13-epi-6-dEB precursor. Overall, this C-H oxidation strategy affords a highly efficient and stereochemically versatile synthesis of the erythromycin core.
Figures



Comment in
-
Total synthesis: expanding the art of synthesis.Nat Chem. 2009 Oct;1(7):519-20. doi: 10.1038/nchem.381. Nat Chem. 2009. PMID: 21378926 No abstract available.
References
-
- Fraunhoffer KJ, Bachovchin DA, White MC. Hydrocarbon oxidation vs. C—C bond-forming approaches for efficient synthesis of oxygenated molecules. Org Lett. 2005;7:223–226. - PubMed
-
- Chen MS, White MC. A predictably selective aliphatic C-H oxidation reaction for complex molecule synthesis. Science. 2007;318:783–787. - PubMed
-
- Das S, Incarvito CD, Crabtree RH, Brudvig GW. Molecular recognition in the selective oxygenation of saturated C-H bonds by a dimanganese catalyst. Science. 2006;312:1941–1943. - PubMed
-
- Yang J, Gabriele B, Belvedere S, Huang Y, Breslow R. Catalytic oxidations of steroid substrates by artificial cytochrome P-450 enzymes. J Org Chem. 2002;67:5057–5067. and references therein. - PubMed
-
- Nicolaou KC, et al. Total synthesis of taxol. Nature. 1994;367:630–634. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/85098522
- PubChem-Substance/85098523
- PubChem-Substance/85098524
- PubChem-Substance/85098525
- PubChem-Substance/85098526
- PubChem-Substance/85098527
- PubChem-Substance/85098528
- PubChem-Substance/85098529
- PubChem-Substance/85098530
- PubChem-Substance/85098531
- PubChem-Substance/85098532
- PubChem-Substance/85098533
- PubChem-Substance/85098534
- PubChem-Substance/85098535
- PubChem-Substance/85098536
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

