Characterization of transcriptome remodeling during cambium formation identifies MOL1 and RUL1 as opposing regulators of secondary growth
- PMID: 21379334
- PMCID: PMC3040665
- DOI: 10.1371/journal.pgen.1001312
Characterization of transcriptome remodeling during cambium formation identifies MOL1 and RUL1 as opposing regulators of secondary growth
Abstract
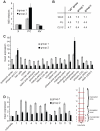
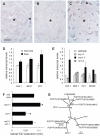
Cell-to-cell communication is crucial for the development of multicellular organisms, especially during the generation of new tissues and organs. Secondary growth--the lateral expansion of plant growth axes--is a highly dynamic process that depends on the activity of the cambium. The cambium is a stem cell-like tissue whose activity is responsible for wood production and, thus, for the establishment of extended shoot and root systems. Attempts to study cambium regulation at the molecular level have been hampered by the limitations of performing genetic analyses in trees and by the difficulty of accessing this tissue in model systems such as Arabidopsis thaliana. Here, we describe the roles of two receptor-like kinases, REDUCED IN LATERAL GROWTH1 (RUL1) and MORE LATERAL GROWTH1 (MOL1), as opposing regulators of cambium activity. Their identification was facilitated by a novel in vitro system in which cambium formation is induced in isolated Arabidopsis stem fragments. By combining this system with laser capture microdissection, we characterized transcriptome remodeling in a tissue- and stage-specific manner and identified series of genes induced during different phases of cambium formation. In summary, we provide a means for investigating cambium regulation in unprecedented depth and present two signaling components that control a process responsible for the accumulation of a large proportion of terrestrial biomass.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Atta R, Laurens L, Boucheron-Dubuisson E, Guivarc'h A, Carnero E, et al. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 2009;57:626–644. - PubMed
-
- Du J, Groover A. Transcriptional regulation of secondary growth and wood formation. J Integr Plant Biol. 2010;52:17–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

