Evolution of copper(II) as a new alkene amination promoter and catalyst
- PMID: 21379363
- PMCID: PMC3046398
- DOI: 10.1016/j.jorganchem.2010.08.041
Evolution of copper(II) as a new alkene amination promoter and catalyst
Abstract
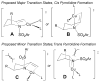
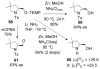
Copper(II) carboxylates and chiral copper(II) triflate·bis(oxazoline) complexes promote and catalyze intramolecular alkene carboamination, diamination and aminooxygenation reactions, creating an array of nitrogen heterocycles. High diastereoselectivity and enantioselectivity can be achieved in these transformations. This account reviews the discovery and development of these useful and interesting reactions.
Figures









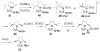

References
-
- Ley SV, Thomas AW. Angew Chem Int Ed Engl. 2003;42:5400–5449. - PubMed
-
- Beletskaya IP, Cheprakov AV. Coord Chem Rev. 2004;248:2337–2364.
-
- Ma D, Cai Q. Acc Chem Res. 2008;41:1450–1460. - PubMed
-
- Evano G, Blanchard N, Toumi M. Chem Rev. 2008;108:3054–3131. - PubMed
-
- Gil MV, Arevalo MJ, Lopez O. Synthesis. 2007:1589–1620.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
