An extra high dose of erythropoietin fails to support the proliferation of erythropoietin dependent cell lines
- PMID: 21380566
- PMCID: PMC3080481
- DOI: 10.1007/s10616-011-9345-x
An extra high dose of erythropoietin fails to support the proliferation of erythropoietin dependent cell lines
Abstract
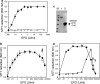
Erythropoietin is responsible for the red blood cell formation by stimulating the proliferation and the differentiation of erythroid precursor cells. Erythropoietin triggers the conformational change in its receptor thereby induces the phosphorylation of JAK2. In this study, we show that an extra high dose of erythropoietin, however, fails to activate the erythropoietin receptor, to stimulate the phosphorylation of JAK2 and to support the cell proliferation of Ep-FDC-P2 cell. Moreover, high dose of EPO also inhibited the proliferation of various erythropoietin-dependent cell lines, suggesting that excess amount of EPO could not trigger the conformational change of the receptor. In the presence of an extra high dose of erythropoietin as well as in the absence of erythropoietin, the cells caused the DNA fragmentation, a typical symptom of apoptosis. The impairment of cell growth and the DNA fragmentation at the extremely high concentration of EPO was rescued by the addition of erythropoietin antibody or soluble form of erythropoietin receptor by titrating the excess erythropoietin. These results suggest that two erythropoietin binding sites on erythropoietin receptor dimer should be occupied by a single erythropoietin molecule for the proper conformational change of the receptor and the signal transduction of erythropoietin, instead, when two erythropoietin binding sites on the receptor are shared by two erythropoietin molecules, it fails to evoke the conformational change of erythropoietin receptor adequate for signal transduction.
Figures




References
-
- Gregoli PA, Bondurant MC. The roles of Bcl-X (L) and apopain in the control of erythropoiesis by erythropoietin. Blood. 1997;90:630–640. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

