Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn's disease: a randomized placebo-controlled trial
- PMID: 21380937
- PMCID: PMC3381945
- DOI: 10.1007/s10620-011-1653-7
Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn's disease: a randomized placebo-controlled trial
Abstract
Background: Endogenous opioid peptides have been shown to play a role in the development and/or perpetuation of inflammation. We hypothesize that the endogenous opioid system is involved in inflammatory bowel disease, and antagonism of the opioid-opioid receptor will lead to reversal of inflammation.
Aims: A randomized double-blind placebo-controlled study was designed to test the efficacy and safety of an opioid antagonist for 12 weeks in adults with active Crohn's disease.
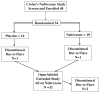
Methods: Forty subjects with active Crohn's disease were enrolled in the study. Randomized patients received daily oral administration of 4.5-mg naltrexone or placebo. Providers and patients were masked to treatment assignment. The primary outcome was the proportion of subjects in each arm with a 70-point decline in Crohn's Disease Activity Index score (CDAI). The secondary outcome included mucosal healing based upon colonoscopy appearance and histology.
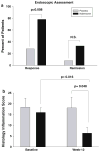
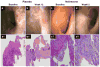
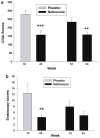
Results: Eighty-eight percent of those treated with naltrexone had at least a 70-point decline in CDAI scores compared to 40% of placebo-treated patients (p = 0.009). After 12 weeks, 78% of subjects treated with naltrexone exhibited an endoscopic response as indicated by a 5-point decline in the Crohn's disease endoscopy index severity score (CDEIS) from baseline compared to 28% response in placebo-treated controls (p = 0.008), and 33% achieved remission with a CDEIS score <6, whereas only 8% of those on placebo showed the same change. Fatigue was the only side effect reported that was significantly greater in subjects receiving placebo.
Conclusions: Naltrexone improves clinical and inflammatory activity of subjects with moderate to severe Crohn's disease compared to placebo-treated controls. Strategies to alter the endogenous opioid system provide promise for the treatment of Crohn's disease.
Conflict of interest statement
Drs. Smith and Zagon have intellectual property rights and have a patent for the use of naltrexone in IBD. This disclosure was provided to all study participants. The statistical analysis of the entire data sets pertaining to efficacy (specifically primary and major secondary efficacy endpoints) and safety (specifically, serious adverse events as defined in federal guidelines) have been independently confirmed by a biostatistician who has no conflict of interest.
Figures
References
-
- Strober W, James SP. The immunopathogenesis of gastrointestinal and hepatobiliary diseases. JAMA. 1992;268:2910–2917. - PubMed
-
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. - PubMed
-
- Gurudu S, Fiocchi C, Katz JA. Inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2002;16:77–90. - PubMed
-
- Targan SR, Murphy LK. Clarifying the causes of Crohn’s. Nat Med. 1995;1:1241–1243. - PubMed
-
- Kelly JK, Sutherland LR. The chronological sequence in the pathology of Crohn’s disease. J Clin Gastroenterol. 1988;10:28–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

