Molecular analysis of neurogenic placode development in a basal ray-finned fish
- PMID: 21381180
- PMCID: PMC4212515
- DOI: 10.1002/dvg.20707
Molecular analysis of neurogenic placode development in a basal ray-finned fish
Abstract
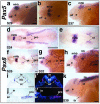
Neurogenic placodes are transient, thickened patches of embryonic vertebrate head ectoderm that give rise to the paired peripheral sense organs and most neurons in cranial sensory ganglia. We present the first analysis of gene expression during neurogenic placode development in a basal actinopterygian (ray-finned fish), the North American paddlefish (Polyodon spathula). Pax3 expression in the profundal placode confirms its homology with the ophthalmic trigeminal placode of amniotes. We report the conservation of expression of Pax2 and Pax8 in the otic and/or epibranchial placodes, Phox2b in epibranchial placode-derived neurons, Sox3 during epibranchial and lateral line placode development, and NeuroD in developing cranial sensory ganglia. We identify Sox3 as a novel marker for developing fields of electrosensory ampullary organs and for ampullary organs themselves. Sox3 is also the first molecular marker for actinopterygian ampullary organs. This is consistent with, though does not prove, a lateral line placode origin for actinopterygian ampullary organs.
Copyright © 2011 Wiley-Liss, Inc.
Figures






References
-
- Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21:2104–2105. - PubMed
-
- Abu-Elmagd M, Ishii Y, Cheung M, Rex M, Le Rouëdec D, Scotting PJ. cSox3 expression and neurogenesis in the epibranchial placodes. Dev Biol. 2001;237:258–269. - PubMed
-
- Alves-Gomes JA. The evolution of electroreception and bioelectrogenesis in teleost fish: a phylogenetic perspective. J Fish Biol. 2001;58:1489–1511.
-
- Baker CVH, Bronner-Fraser M. Establishing neuronal identity in vertebrate neurogenic placodes. Development. 2000;127:3045–3056. - PubMed
-
- Baker CVH, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

