The developing oligodendrocyte: key cellular target in brain injury in the premature infant
- PMID: 21382469
- PMCID: PMC3099053
- DOI: 10.1016/j.ijdevneu.2011.02.012
The developing oligodendrocyte: key cellular target in brain injury in the premature infant
Abstract
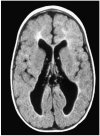
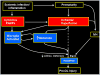
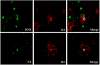
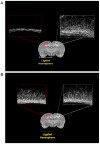
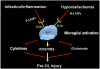
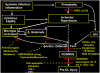
Brain injury in the premature infant, a problem of enormous importance, is associated with a high risk of neurodevelopmental disability. The major type of injury involves cerebral white matter and the principal cellular target is the developing oligodendrocyte. The specific phase of the oligodendroglial lineage affected has been defined from study of both human brain and experimental models. This premyelinating cell (pre-OL) is vulnerable because of a series of maturation-dependent events. The pathogenesis of pre-OL injury relates to operation of two upstream mechanisms, hypoxia-ischemia and systemic infection/inflammation, both of which are common occurrences in premature infants. The focus of this review and of our research over the past 15-20 years has been the cellular and molecular bases for the maturation-dependent vulnerability of the pre-OL to the action of the two upstream mechanisms. Three downstream mechanisms have been identified, i.e., microglial activation, excitotoxicity and free radical attack. The work in both experimental models and human brain has identified a remarkable confluence of maturation-dependent factors that render the pre-OL so exquisitely vulnerable to these downstream mechanisms. Most importantly, elucidation of these factors has led to delineation of a series of potential therapeutic interventions, which in experimental models show marked protective properties. The critical next step, i.e., clinical trials in the living infant, is now on the horizon.
Copyright © 2011 ISDN. Published by Elsevier Ltd. All rights reserved.
Figures








Republished in
-
Reprint of "The developing oligodendrocyte: key cellular target in brain injury in the premature infant".Int J Dev Neurosci. 2011 Oct;29(6):565-82. doi: 10.1016/j.ijdevneu.2011.07.008. Epub 2011 Jul 23. Int J Dev Neurosci. 2011. PMID: 21802506
References
-
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. - PubMed
-
- Agresti C, D’Urso D, Levi G. Reversible inhibitory effects of interferon-γ- and tumour necrosis factor-α on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–1116. - PubMed
-
- Alberdi E, Sanchez-Gomez MV, Marino A, Matute C. Ca(2+) influx through AMPA or kainate receptors alone is sufficient to initiate excitotoxicity in cultured oligodendrocytes. Neurobiol Dis. 2002;2:234–243. - PubMed
-
- Albrecht P, Lewerenz J, Dittmer S, Noack R, Maher P, Methner A. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol Disord Drug Targets. 2010;9:373–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

