Caveolin-1 mediates Fas-BID signaling in hyperoxia-induced apoptosis
- PMID: 21382479
- PMCID: PMC4134776
- DOI: 10.1016/j.freeradbiomed.2011.02.031
Caveolin-1 mediates Fas-BID signaling in hyperoxia-induced apoptosis
Abstract
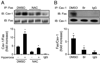
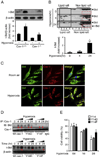
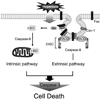
Fas-mediated apoptosis is a crucial cellular event. Fas, the Fas-associated death domain, and caspase 8 form the death-inducing signaling complex (DISC). Activated caspase 8 mediates the extrinsic pathways and cleaves cytosolic BID. Truncated BID (tBID) translocates to the mitochondria, facilitates the release of cytochrome c, and activates the intrinsic pathways. However, the mechanism causing these DISC components to aggregate and form the complex remains unclear. We found that Cav-1 regulated Fas signaling and mediated the communication between extrinsic and intrinsic pathways. Shortly after hyperoxia (4 h), the colocalization and interaction of Cav-1 and Fas increased, followed by Fas multimer and DISC formation. Deletion of Cav-1 (Cav-1-/-) disrupted DISC formation. Further, Cav-1 interacted with BID. Mutation of Cav-1 Y14 tyrosine to phenylalanine (Y14F) disrupted the hyperoxia-induced interaction between BID and Cav-1 and subsequently yielded a decreased level of tBID and resistance to hyperoxia-induced apoptosis. The reactive oxygen species (ROS) scavenger N-acetylcysteine decreased the Cav-1-Fas interaction. Deletion of glutathione peroxidase-2 using siRNA aggravated the BID-Cav-1 interaction and tBID formation. Taken together, these results indicate that Cav-1 regulates hyperoxia/ROS-induced apoptosis through interactions with Fas and BID, probably via Fas palmitoylation and Cav-1 Y14 phosphorylation, respectively.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:305–1308. - PubMed
-
- Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Human Fas ligand: gene structure, chromosomal location and species specificity. Int. Immunol. 1994;6:1567–1574. - PubMed
-
- Ogasawara J, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. - PubMed
-
- Jones RA, et al. Fas-mediated apoptosis in mouse hepatocytes involves the processing and activation of caspases. Hepatology. 1998;27:1632–1642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

