Synaptic integration gradients in single cortical pyramidal cell dendrites
- PMID: 21382549
- PMCID: PMC6420135
- DOI: 10.1016/j.neuron.2011.02.006
Synaptic integration gradients in single cortical pyramidal cell dendrites
Abstract
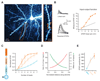
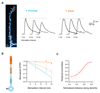
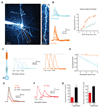
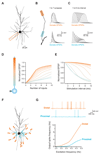
Cortical pyramidal neurons receive thousands of synaptic inputs arriving at different dendritic locations with varying degrees of temporal synchrony. It is not known if different locations along single cortical dendrites integrate excitatory inputs in different ways. Here we have used two-photon glutamate uncaging and compartmental modeling to reveal a gradient of nonlinear synaptic integration in basal and apical oblique dendrites of cortical pyramidal neurons. Excitatory inputs to the proximal dendrite sum linearly and require precise temporal coincidence for effective summation, whereas distal inputs are amplified with high gain and integrated over broader time windows. This allows distal inputs to overcome their electrotonic disadvantage, and become surprisingly more effective than proximal inputs at influencing action potential output. Thus, single dendritic branches can already exhibit nonuniform synaptic integration, with the computational strategy shifting from temporal coding to rate coding along the dendrite.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Abeles M. Corticonics: neural circuits of the cerebral cortex. Cambridge, UK: Cambridge University Press; 1991.
-
- Andersen P, Morris R, Amaral D, Bliss TV, O'Keefe J. The Hippocampus Book. New York: Oxford University Press; 2006.
-
- Berger T, Larkum ME, Lüscher HR. High I(h) channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85:855–68. - PubMed
-
- Branco T, Häusser M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol. 2010;20:494–502. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

