The cerebrospinal fluid provides a proliferative niche for neural progenitor cells
- PMID: 21382550
- PMCID: PMC3085909
- DOI: 10.1016/j.neuron.2011.01.023
The cerebrospinal fluid provides a proliferative niche for neural progenitor cells
Abstract
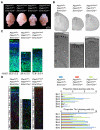
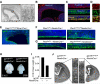
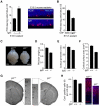
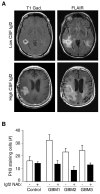
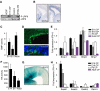
Cortical development depends on the active integration of cell-autonomous and extrinsic cues, but the coordination of these processes is poorly understood. Here, we show that the apical complex protein Pals1 and Pten have opposing roles in localizing the Igf1R to the apical, ventricular domain of cerebral cortical progenitor cells. We found that the cerebrospinal fluid (CSF), which contacts this apical domain, has an age-dependent effect on proliferation, much of which is attributable to Igf2, but that CSF contains other signaling activities as well. CSF samples from patients with glioblastoma multiforme show elevated Igf2 and stimulate stem cell proliferation in an Igf2-dependent manner. Together, our findings demonstrate that the apical complex couples intrinsic and extrinsic signaling, enabling progenitors to sense and respond appropriately to diffusible CSF-borne signals distributed widely throughout the brain. The temporal control of CSF composition may have critical relevance to normal development and neuropathological conditions.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Ayer-le Lievre C, Stahlbom PA, Sara VR. Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus. Development. 1991;111:105–115. - PubMed
-
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. - PubMed
-
- Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bosse M, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. - PubMed
-
- Binoux M, Lassarre C, Gourmelen M. Specific assay for insulin-like growth factor (IGF) II using the IGF binding proteins extracted from human cerebrospinal fluid. J Clin Endocrinol Metab. 1986;63:1151–1155. - PubMed
-
- Bondy C, Werner H, Roberts CT, Jr., LeRoith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neuroscience. 1992;46:909–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD029178/HD/NICHD NIH HHS/United States
- R01 HD008299/HD/NICHD NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- P30 NS045892/NS/NINDS NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R37 NS035129/NS/NINDS NIH HHS/United States
- R01 NS035129/NS/NINDS NIH HHS/United States
- R01 NS048868/NS/NINDS NIH HHS/United States
- R01 HD029178/HD/NICHD NIH HHS/United States
- P30 HD18655/HD/NICHD NIH HHS/United States
- R01 NS032457/NS/NINDS NIH HHS/United States
- K99NS072192/NS/NINDS NIH HHS/United States
- K99 NS072192/NS/NINDS NIH HHS/United States
- R29 HD029178/HD/NICHD NIH HHS/United States
- 3 R01 NS032457/NS/NINDS NIH HHS/United States
- R01 HD042182/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

