[Abstinence rates with varenicline compared to bupropion and nicotine replacement therapy for quitting smoking in primary care]
- PMID: 21382649
- PMCID: PMC7024938
- DOI: 10.1016/j.aprim.2010.09.010
[Abstinence rates with varenicline compared to bupropion and nicotine replacement therapy for quitting smoking in primary care]
Abstract
Objective: The objective of this study was to estimate the continuous abstinence rates of varenicline, bupropion and nicotine replacement therapy (NRT) in smoking cessation in 2 Primary Care (PC) External Support Units.
Design: Observational, multicentre, longitudinal study using a retrospective review of medical records.
Setting: Six Primary Care Centres.
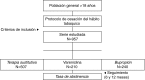
Participants: Patients > 18 years, who began smoking cessation treatment between 1/01/2006 and 1/12/2008 with varenicline, bupropion or NRT were included in the analysis. CONTINUATION: Patient follow-up was conducted from time-baseline (day 1) and assessed at 6 and 12 months.
Main variables: comorbidities, effectiveness (continuous abstinence) and pharmacological tolerability.
Statistical analysis: logistic regression models and Kaplan-Maier survival curves; P<.05.
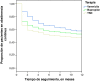
Results: A total of 957 smokers treated with NRT (53.0%), bupropion (25.1%) and varenicline (21.9%) were included in the analysis. Mean age: 47.6 (11.3) years; 58.6% men. 32.0% of smokers attended due to physical dependence. Average duration of smoking: 19.5 (6.7) years. At 6 months, 61.2% (95% CI: 54.6-67.8%) of participants in the varenicline group continuously abstained from smoking compared with 56.9% (95% CI: 50.6-63.2%) in the bupropion group, and 52.3% (95% CI: 48.0-56.6%) in the NRT group; P=.003. At 12 months, the rate of continuous abstinence was 57.4% (95% CI: 50.7-64.1%) in the varenicline group compared with 52.9% (95% CI: 46.6-59.2%) in the bupropion group and 47.1% (95% CI: 42.8-51.4%) in the NRT group; P=.002.
Conclusion: One year-follow up results suggest that varenicline is an appropriate alternative compared with bupropion and NRT on smoking cessation in the PC setting.
Objetivo: Determinar las tasas de abstinencia continua conseguidas con vareniclina, bupropión y terapia de sustitución nicotínica (TSN) en la cesación del consumo de tabaco en 2 unidades externas de apoyo a atención primaria (AP).
Diseño: Estudio observacional, multicéntrico, longitudinal, a partir de la revisión retrospectiva de registros médicos.
Emplazamiento: Seis centros de AP.
Participantes: Se incluyeron pacientes > 18 años que iniciaron tratamiento de cesación del hábito tabáquico entre 1/01/2006 y 31/12/2008 con vareniclina, bupropión o TSN.
Mediciones principales: Principales medidas: sociodemográficas, comorbilidad, efectividad (abstinencia continua) y tolerabilidad farmacológica. Se evaluó la abstinencia a los 6 y 12 meses de seguimiento. Análisis estadístico: modelos de regresión logística y curva de supervivencia de Kaplan-Meier.
Resultados: Se incluyeron 957 pacientes fumadores, tratados con TSN (53,0%), bupropión (25,1%) y vareniclina (21,9%). Su media de edad era 47,6 (DE: 11,3) años, y el 58,6% eran hombres. Un 32,0% acudieron por dependencia física y el tiempo medio de fumadores fue de 19,5 (DE: 6,7) años. A los 6 meses, la abstinencia continua con vareniclina fue del 61,2% (IC: 54,6-67,8%), con bupropión del 56,9% (IC: 50,6-63,2%) y con TSN del 52,3% (IC: 48,0-56,6%); p = 0,003. A los 12 meses, fue del 57,4% (IC: 50,7-64,1%), 52,9% (IC: 46,6-59,2%) y 47,1% (IC: 42,8-51,4%), respectivamente, p = 0,002.
Conclusiones: Los resultados tras un año de seguimiento sugieren que vareniclina es una alternativa adecuada frente al bupropión y la TSN en la cesación del tabaco en el ámbito de la AP.
Copyright © 2010 Elsevier España, S.L. All rights reserved.
Figures
References
-
- World Health Organization . World Health Organization; Warsaw: 2002. The European report on tobacco control policy. WHO European ministerial conference for a tobacco-free Europe.
-
- Burns D.M. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46:11–29. - PubMed
-
- Teo K.K., Ounpuu S., Hawken S., Pandey M.R., Valentin V., Hunt D. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647–658. - PubMed
-
- Rahman M.M., Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol. 2007;5:276–292. - PubMed
-
- Lavi S., Prasad A., Yang E.H., Mathew V., Simari R.D., Rihal C.S. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation. 2007;115:2621–2627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

