S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation
- PMID: 21382888
- PMCID: PMC3072335
- DOI: 10.1161/CIRCULATIONAHA.110.985523
S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation
Abstract
Background: S100A9 is constitutively expressed in neutrophils, dendritic cells, and monocytes; is associated with acute and chronic inflammatory conditions; and is implicated in obesity and cardiovascular disease in humans. Most of the constitutively secreted S100A9 is derived from myeloid cells. A recent report demonstrated that mice deficient in S100A9 exhibit reduced atherosclerosis compared with controls and suggested that this effect was due in large part to loss of S100A9 in bone marrow-derived cells.
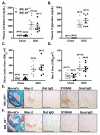
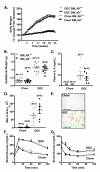
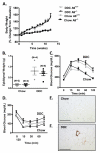
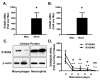
Methods and results: To directly investigate the role of bone marrow-derived S100A9 in atherosclerosis and insulin resistance in mice, low-density lipoprotein receptor-deficient, S100A9-deficient bone marrow chimeras were generated. Neither atherosclerosis nor insulin resistance was reduced in S100A9-deficient chimeras fed a diet rich in fat and carbohydrates. To investigate the reason for this lack of effect, myeloid cells were isolated from the peritoneal cavity or bone marrow. S100A9-deficient neutrophils exhibited a reduced secretion of cytokines in response to toll-like receptor-4 stimulation. In striking contrast, S100A9-deficient dendritic cells showed an exacerbated release of cytokines after toll-like receptor stimulation. Macrophages rapidly lost S100A9 expression during maturation; hence, S100A9 deficiency did not affect the inflammatory status of macrophages.
Conclusions: S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells. The effect of S100A9 deficiency on atherosclerosis and other inflammatory diseases is therefore predicted to depend on the relative contribution of these cell types at different stages of disease progression. Furthermore, S100A9 expression in nonmyeloid cells is likely to contribute to atherosclerosis.
Figures

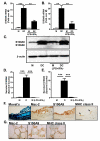

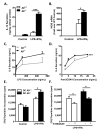
References
-
- Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker PM, Simon DI. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113:2278–2284. - PubMed
-
- Frosch M, Vogl T, Seeliger S, Wulffraat N, Kuis W, Viemann D, Foell D, Sorg C, Sunderkotter C, Roth J. Expression of myeloid-related proteins 8 and 14 in systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2003;48:2622–2626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007828/HL/NHLBI NIH HHS/United States
- R01 HL062887/HL/NHLBI NIH HHS/United States
- HL097365/HL/NHLBI NIH HHS/United States
- HL079382/HL/NHLBI NIH HHS/United States
- AI073441/AI/NIAID NIH HHS/United States
- R01 AI081948/AI/NIAID NIH HHS/United States
- P01 HL092969/HL/NHLBI NIH HHS/United States
- HL062887/HL/NHLBI NIH HHS/United States
- P01 HL030086/HL/NHLBI NIH HHS/United States
- HL092969/HL/NHLBI NIH HHS/United States
- R01 HL097365/HL/NHLBI NIH HHS/United States
- HL030086/HL/NHLBI NIH HHS/United States
- R01 HL079382/HL/NHLBI NIH HHS/United States
- T32 HL07828/HL/NHLBI NIH HHS/United States
- R01 AI073441/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

