Results from a dose-response study using 3,3'-diindolylmethane in the K14-HPV16 transgenic mouse model: cervical histology
- PMID: 21383027
- PMCID: PMC3107883
- DOI: 10.1158/1940-6207.CAPR-10-0369
Results from a dose-response study using 3,3'-diindolylmethane in the K14-HPV16 transgenic mouse model: cervical histology
Abstract
The human papilloma virus is the major cause of cervical cancer. Viral infection initiates cervical intraepithelial neoplasia, which progresses through several stages to cervical cancer. The objective of this study is to identify the minimum effective dose of diindolylmethane that prevents the progression from cervical dysplasia to carcinoma in situ. We document cervical histology in K14-HPV16 mice receiving different doses of diindolylmethane. Urinary diindolylmethane concentrations are reported. Diindolylmethane could enhance the efficacy of human papilloma virus vaccines, creating a new therapeutic use for these vaccines in women already infected with the virus. Five doses (0-2,500 ppm) of diindolylmethane were incorporated into each mouse diet. The reproductive tract was serially sectioned and urine was obtained for analysis of urinary diindolylmethane. The results indicate that 62% of mice receiving 1,000 ppm diindolylmethane remained dysplasia-free after 20 weeks compared with 16% of mice receiving no diindolylmethane and 18% receiving 500 ppm; 1,000 ppm of 3,3'-diindolylmethane in the diet completely suppressed the development of cervical cancer. Urinary diindolylmethane levels increased significantly as diindolylmethane in food increased. These findings imply usefulness for diindolylmethane in the search to prevent cervical cancer when used in combination with prophylactic or therapeutic vaccines.
Figures


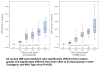
References
-
- U.S. Department of Health and Human Services - National Cancer Institute, (NCI) Cancer Incidence – Surveillance, Epidemiology, and End Results (SEER) Registries Research Data. Bethesda, Maryland: National Cancer Institute, Cancer Statistics Branch; Apr 15, 2010.
-
- Division of STD Prevention. Prevention of genital HPV infection and sequelae: Report of an external consultants’ meeting. Atlanta, GA: Centers for Disease Control and Prevention; 1999.
-
- Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347(21):1645–51. - PubMed
-
- Villa L, Costa R, Petta C, Andrade R, Ault K, Giuliano A, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. The Lancet Oncology. 2005;6(5):271–278. - PubMed

