Legionella pneumophila type II secretion dampens the cytokine response of infected macrophages and epithelia
- PMID: 21383054
- PMCID: PMC3088156
- DOI: 10.1128/IAI.01077-10
Legionella pneumophila type II secretion dampens the cytokine response of infected macrophages and epithelia
Abstract
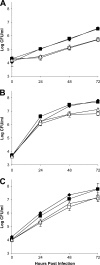
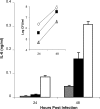
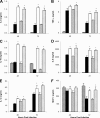
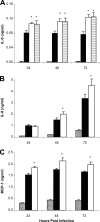
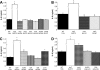
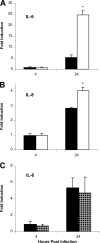
The type II secretion (T2S) system of Legionella pneumophila is required for the ability of the bacterium to grow within the lungs of A/J mice. By utilizing mutants lacking T2S (lsp), we now document that T2S promotes the intracellular infection of both multiple types of macrophages and lung epithelia. Following infection of macrophages, lsp mutants (but not a complemented mutant) elicited significantly higher levels of interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), IL-10, IL-8, IL-1β, and MCP-1 within tissue culture supernatants. A similar result was obtained with infected lung epithelial cell lines and the lungs of infected A/J mice. Infection with a mutant specifically lacking the T2S-dependent ProA protease (but not a complemented proA mutant) resulted in partial elevation of cytokine levels. These data demonstrate that the T2S system of L. pneumophila dampens the cytokine/chemokine output of infected host cells. Upon quantitative reverse transcription (RT)-PCR analysis of infected host cells, an lspF mutant, but not the proA mutant, produced significantly higher levels of cytokine transcripts, implying that some T2S-dependent effectors dampen signal transduction and transcription but that others, such as ProA, act at a posttranscriptional step in cytokine expression. In summary, the impact of T2S on lung infection is a combination of at least three factors: the promotion of growth in macrophages, the facilitation of growth in epithelia, and the dampening of the chemokine and cytokine output from infected host cells. To our knowledge, these data are the first to identify a link between a T2S system and the modulation of immune factors following intracellular infection.
Figures





Similar articles
-
The Type II Secretion System of Legionella pneumophila Dampens the MyD88 and Toll-Like Receptor 2 Signaling Pathway in Infected Human Macrophages.Infect Immun. 2017 Mar 23;85(4):e00897-16. doi: 10.1128/IAI.00897-16. Print 2017 Apr. Infect Immun. 2017. PMID: 28138020 Free PMC article.
-
Multiple Legionella pneumophila Type II secretion substrates, including a novel protein, contribute to differential infection of the amoebae Acanthamoeba castellanii, Hartmannella vermiformis, and Naegleria lovaniensis.Infect Immun. 2013 May;81(5):1399-410. doi: 10.1128/IAI.00045-13. Epub 2013 Feb 19. Infect Immun. 2013. PMID: 23429532 Free PMC article.
-
Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires' disease pneumonia.Infect Immun. 2004 Jan;72(1):310-21. doi: 10.1128/IAI.72.1.310-321.2004. Infect Immun. 2004. PMID: 14688110 Free PMC article.
-
Type II secretion and Legionella virulence.Curr Top Microbiol Immunol. 2013;376:81-102. doi: 10.1007/82_2013_339. Curr Top Microbiol Immunol. 2013. PMID: 23900831 Review.
-
[Host-pathogen interaction of Legionella pneumophila].Nihon Saikingaku Zasshi. 2014;69(3):503-11. doi: 10.3412/jsb.69.503. Nihon Saikingaku Zasshi. 2014. PMID: 25186641 Review. Japanese.
Cited by
-
The major facilitator superfamily-type protein LbtC promotes the utilization of the legiobactin siderophore by Legionella pneumophila.Microbiology (Reading). 2012 Mar;158(Pt 3):721-735. doi: 10.1099/mic.0.055533-0. Epub 2011 Dec 8. Microbiology (Reading). 2012. PMID: 22160401 Free PMC article.
-
Zinc Metalloprotease ProA from Legionella pneumophila Inhibits the Pro-Inflammatory Host Response by Degradation of Bacterial Flagellin.Biomolecules. 2022 Apr 22;12(5):624. doi: 10.3390/biom12050624. Biomolecules. 2022. PMID: 35625552 Free PMC article.
-
Two novel functions of hyaluronidase from Streptococcus agalactiae are enhanced intracellular survival and inhibition of proinflammatory cytokine expression.Infect Immun. 2014 Jun;82(6):2615-25. doi: 10.1128/IAI.00022-14. Epub 2014 Apr 7. Infect Immun. 2014. PMID: 24711564 Free PMC article.
-
Type II Secretion-Dependent Aminopeptidase LapA and Acyltransferase PlaC Are Redundant for Nutrient Acquisition during Legionella pneumophila Intracellular Infection of Amoebas.mBio. 2018 Apr 17;9(2):e00528-18. doi: 10.1128/mBio.00528-18. mBio. 2018. PMID: 29666285 Free PMC article.
-
Nuclease activity of Legionella pneumophila Cas2 promotes intracellular infection of amoebal host cells.Infect Immun. 2015 Mar;83(3):1008-18. doi: 10.1128/IAI.03102-14. Epub 2014 Dec 29. Infect Immun. 2015. PMID: 25547789 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

