Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy
- PMID: 21383056
- PMCID: PMC3058575
- DOI: 10.1084/jem.20100269
Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy
Erratum in
- J Exp Med. 2011 Apr 11:208(4):869
Abstract
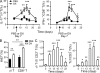
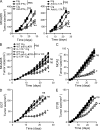
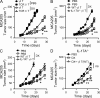
By triggering immunogenic cell death, some anticancer compounds, including anthracyclines and oxaliplatin, elicit tumor-specific, interferon-γ-producing CD8(+) αβ T lymphocytes (Tc1 CTLs) that are pivotal for an optimal therapeutic outcome. Here, we demonstrate that chemotherapy induces a rapid and prominent invasion of interleukin (IL)-17-producing γδ (Vγ4(+) and Vγ6(+)) T lymphocytes (γδ T17 cells) that precedes the accumulation of Tc1 CTLs within the tumor bed. In T cell receptor δ(-/-) or Vγ4/6(-/-) mice, the therapeutic efficacy of chemotherapy was compromised, no IL-17 was produced by tumor-infiltrating T cells, and Tc1 CTLs failed to invade the tumor after treatment. Although γδ T17 cells could produce both IL-17A and IL-22, the absence of a functional IL-17A-IL-17R pathway significantly reduced tumor-specific T cell responses elicited by tumor cell death, and the efficacy of chemotherapy in four independent transplantable tumor models. Adoptive transfer of γδ T cells restored the efficacy of chemotherapy in IL-17A(-/-) hosts. The anticancer effect of infused γδ T cells was lost when they lacked either IL-1R1 or IL-17A. Conventional helper CD4(+) αβ T cells failed to produce IL-17 after chemotherapy. We conclude that γδ T17 cells play a decisive role in chemotherapy-induced anticancer immune responses.
Figures




References
-
- Couzi L., Levaillant Y., Jamai A., Pitard V., Lassalle R., Martin K., Garrigue I., Hawchar O., Siberchicot F., Moore N., et al. 2010. Cytomegalovirus-induced gammadelta T cells associate with reduced cancer risk after kidney transplantation. J. Am. Soc. Nephrol. 21:181–188 10.1681/ASN.2008101072 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

