VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses
- PMID: 21383057
- PMCID: PMC3058578
- DOI: 10.1084/jem.20100619
VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses
Abstract
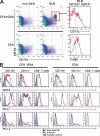
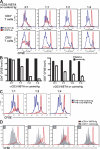
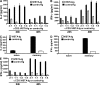
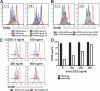
The immunoglobulin (Ig) superfamily consists of many critical immune regulators, including the B7 family ligands and receptors. In this study, we identify a novel and structurally distinct Ig superfamily inhibitory ligand, whose extracellular domain bears homology to the B7 family ligand PD-L1. This molecule is designated V-domain Ig suppressor of T cell activation (VISTA). VISTA is primarily expressed on hematopoietic cells, and VISTA expression is highly regulated on myeloid antigen-presenting cells (APCs) and T cells. A soluble VISTA-Ig fusion protein or VISTA expression on APCs inhibits T cell proliferation and cytokine production in vitro. A VISTA-specific monoclonal antibody interferes with VISTA-induced suppression of T cell responses by VISTA-expressing APCs in vitro. Furthermore, anti-VISTA treatment exacerbates the development of the T cell-mediated autoimmune disease experimental autoimmune encephalomyelitis in mice. Finally, VISTA overexpression on tumor cells interferes with protective antitumor immunity in vivo in mice. These findings show that VISTA, a novel immunoregulatory molecule, has functional activities that are nonredundant with other Ig superfamily members and may play a role in the development of autoimmunity and immune surveillance in cancer.
Figures



References
-
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA164225/CA/NCI NIH HHS/United States
- R01 GM096041/GM/NIGMS NIH HHS/United States
- R01 AI048667/AI/NIAID NIH HHS/United States
- G0802651/MRC_/Medical Research Council/United Kingdom
- R01 AI098007/AI/NIAID NIH HHS/United States
- U54 GM094662/GM/NIGMS NIH HHS/United States
- U01 GM094665/GM/NIGMS NIH HHS/United States
- R01 AI007289/AI/NIAID NIH HHS/United States
- AI048667-06A1/AI/NIAID NIH HHS/United States
- 1U54GM094662/GM/NIGMS NIH HHS/United States
- 5R01AI007289/AI/NIAID NIH HHS/United States
- 1U01GM094665/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

