Diversity through phosphine catalysis identifies octahydro-1,6-naphthyridin-4-ones as activators of endothelium-driven immunity
- PMID: 21383121
- PMCID: PMC3084088
- DOI: 10.1073/pnas.1015254108
Diversity through phosphine catalysis identifies octahydro-1,6-naphthyridin-4-ones as activators of endothelium-driven immunity
Abstract
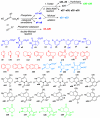
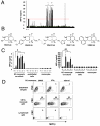
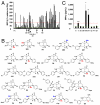
The endothelium plays a critical role in promoting inflammation in cardiovascular disease and other chronic inflammatory conditions, and many small-molecule screens have sought to identify agents that prevent endothelial cell activation. Conversely, an augmented immune response can be protective against microbial pathogens and in cancer immunotherapy. Yet, small-molecule screens to identify agents that induce endothelial cell activation have not been reported. In this regard, a bioassay was developed that identifies activated endothelium by its capacity to trigger macrophage inflammatory protein 1 beta from primary monocytes. Subsequently, a 642-compound library of 39 distinctive scaffolds generated by a diversity-oriented synthesis based on the nucleophilic phosphine catalysis was screened for small molecules that activated the endothelium. Among the active compounds identified, the major classes were synthesized through the sequence of phosphine-catalyzed annulation, Tebbe reaction, Diels-Alder reaction, and in some cases, hydrolysis. Ninety-six analogs of one particular class of compounds, octahydro-1,6-naphthyridin-4-ones, were efficiently prepared by a solid-phase split-and-pool technique and by solution phase analog synthesis. Structure-function analysis combined with transcriptional profiling of active and inactive octahydro-1,6-naphthyridin-4-one analogs identified inflammatory gene networks induced exclusively by the active compound. The identification of a family of chemical probes that augment innate immunity through endothelial cell activation provides a framework for understanding gene networks involved in endothelial inflammation as well as the development of novel endothelium-driven immunotherapeutic agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Schreiber SL, Kapoor TM, Wess G. Chemical Biology: From Small Molecules to Systems Biology and Drug Design. Weinheim, Germany: Wiley-VCH; 2008.
-
- Schreiber SL. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science. 2000;287:1964–1969. - PubMed
-
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. - PubMed
-
- Strausberg RL, Schreiber SL. From knowing to controlling: A path from genomics to drugs using small molecule probes. Science. 2003;300:294–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

