Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma
- PMID: 21383194
- PMCID: PMC3064324
- DOI: 10.1073/pnas.1019055108
Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma
Abstract
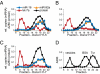
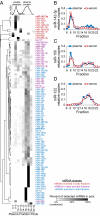
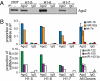
MicroRNAs (miRNAs) circulate in the bloodstream in a highly stable, extracellular form and are being developed as blood-based biomarkers for cancer and other diseases. However, the mechanism underlying their remarkable stability in the RNase-rich environment of blood is not well understood. The current model in the literature posits that circulating miRNAs are protected by encapsulation in membrane-bound vesicles such as exosomes, but this has not been systematically studied. We used differential centrifugation and size-exclusion chromatography as orthogonal approaches to characterize circulating miRNA complexes in human plasma and serum. We found, surprisingly, that the majority of circulating miRNAs cofractionated with protein complexes rather than with vesicles. miRNAs were also sensitive to protease treatment of plasma, indicating that protein complexes protect circulating miRNAs from plasma RNases. Further characterization revealed that Argonaute2 (Ago2), the key effector protein of miRNA-mediated silencing, was present in human plasma and eluted with plasma miRNAs in size-exclusion chromatography. Furthermore, immunoprecipitation of Ago2 from plasma readily recovered non-vesicle-associated plasma miRNAs. The majority of miRNAs studied copurified with the Ago2 ribonucleoprotein complex, but a minority of specific miRNAs associated predominantly with vesicles. Our results reveal two populations of circulating miRNAs and suggest that circulating Ago2 complexes are a mechanism responsible for the stability of plasma miRNAs. Our study has important implications for the development of biomarker approaches based on capture and analysis of circulating miRNAs. In addition, identification of extracellular Ago2-miRNA complexes in plasma raises the possibility that cells release a functional miRNA-induced silencing complex into the circulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

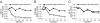
Comment in
-
Die hard: resilient RNAs in the blood.Nat Rev Mol Cell Biol. 2021 Jun;22(6):373. doi: 10.1038/s41580-021-00355-9. Epub 2021 Mar 4. Nat Rev Mol Cell Biol. 2021. PMID: 33664511 No abstract available.
References
-
- Chen X, et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. - PubMed
-
- Chim SS, et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54:482–490. - PubMed
-
- Lawrie CH, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

