Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission
- PMID: 21383202
- PMCID: PMC3064317
- DOI: 10.1073/pnas.1102714108
Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission
Abstract
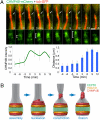
The final stage of cytokinesis is abscission, the cutting of the narrow membrane bridge connecting two daughter cells. The endosomal sorting complex required for transport (ESCRT) machinery is required for cytokinesis, and ESCRT-III has membrane scission activity in vitro, but the role of ESCRTs in abscission has been undefined. Here, we use structured illumination microscopy and time-lapse imaging to dissect the behavior of ESCRTs during abscission. Our data reveal that the ESCRT-I subunit tumor-susceptibility gene 101 (TSG101) and the ESCRT-III subunit charged multivesicular body protein 4b (CHMP4B) are sequentially recruited to the center of the intercellular bridge, forming a series of cortical rings. Late in cytokinesis, however, CHMP4B is acutely recruited to the narrow constriction site where abscission occurs. The ESCRT disassembly factor vacuolar protein sorting 4 (VPS4) follows CHMP4B to this site, and cell separation occurs immediately. That arrival of ESCRT-III and VPS4 correlates both spatially and temporally with the abscission event suggests a direct role for these proteins in cytokinetic membrane abscission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. - PubMed
-
- Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: From parts list to mechanisms. Annu Rev Biochem. 2006;75:543–566. - PubMed
-
- Steigemann P, Gerlich DW. Cytokinetic abscission: Cellular dynamics at the midbody. Trends Cell Biol. 2009;19:606–616. - PubMed
-
- Sagona AP, Stenmark H. Cytokinesis and cancer. FEBS Lett. 2010;584:2652–2661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

