Gene x disease interaction on orbitofrontal gray matter in cocaine addiction
- PMID: 21383264
- PMCID: PMC3127452
- DOI: 10.1001/archgenpsychiatry.2011.10
Gene x disease interaction on orbitofrontal gray matter in cocaine addiction
Abstract
Context: Long-term cocaine use has been associated with structural deficits in brain regions having dopamine-receptive neurons. However, the concomitant use of other drugs and common genetic variability in monoamine regulation present additional structural variability.
Objective: To examine variations in gray matter volume (GMV) as a function of lifetime drug use and the genotype of the monoamine oxidase A gene, MAOA, in men with cocaine use disorders (CUD) and healthy male controls.
Design: Cross-sectional comparison.
Setting: Clinical Research Center at Brookhaven National Laboratory.
Patients: Forty individuals with CUD and 42 controls who underwent magnetic resonance imaging to assess GMV and were genotyped for the MAOA polymorphism (categorized as high- and low-repeat alleles).
Main outcome measures: The impact of cocaine addiction on GMV, tested by (1) comparing the CUD group with controls, (2) testing diagnosis × MAOA interactions, and (3) correlating GMV with lifetime cocaine, alcohol, and cigarette smoking, and testing their unique contribution to GMV beyond other factors.
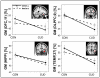
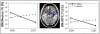
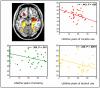
Results: (1) Individuals with CUD had reductions in GMV in the orbitofrontal, dorsolateral prefrontal, and temporal cortex and the hippocampus compared with controls. (2) The orbitofrontal cortex reductions were uniquely driven by CUD with low- MAOA genotype and by lifetime cocaine use. (3) The GMV in the dorsolateral prefrontal cortex and hippocampus was driven by lifetime alcohol use beyond the genotype and other pertinent variables.
Conclusions: Long-term cocaine users with the low-repeat MAOA allele have enhanced sensitivity to gray matter loss, specifically in the orbitofrontal cortex, indicating that this genotype may exacerbate the deleterious effects of cocaine in the brain. In addition, long-term alcohol use is a major contributor to gray matter loss in the dorsolateral prefrontal cortex and hippocampus, and is likely to further impair executive function and learning in cocaine addiction.
Figures
References
-
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. - PubMed
-
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–325. - PubMed
-
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? The American journal of psychiatry. 2007;164(1):43–51. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

