Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling
- PMID: 21383288
- PMCID: PMC3157968
- DOI: 10.1200/JCO.2010.33.2312
Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling
Erratum in
-
Erratum: Dissecting Therapeutic Resistance to RAF Inhibition in Melanoma by Tumor Genomic Profiling.J Clin Oncol. 2024 Mar 10;42(8):976. doi: 10.1200/JCO.24.00017. Epub 2024 Feb 1. J Clin Oncol. 2024. PMID: 38301177 No abstract available.
Expression of concern in
-
Withdrawn: Expression of Concern: Dissecting Therapeutic Resistance to RAF Inhibition in Melanoma by Tumor Genomic Profiling.J Clin Oncol. 2025 Mar;43(7):e1. doi: 10.1200/JCO.24.00161. Epub 2024 Feb 23. J Clin Oncol. 2025. PMID: 38394605 No abstract available.
Abstract
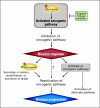
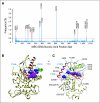
A detailed understanding of the mechanisms by which tumors acquire resistance to targeted anticancer agents should speed the development of treatment strategies with lasting clinical efficacy. RAF inhibition in BRAF-mutant melanoma exemplifies the promise and challenge of many targeted drugs; although response rates are high, resistance invariably develops. Here, we articulate overarching principles of resistance to kinase inhibitors, as well as a translational approach to characterize resistance in the clinical setting through tumor mutation profiling. As a proof of principle, we performed targeted, massively parallel sequencing of 138 cancer genes in a tumor obtained from a patient with melanoma who developed resistance to PLX4032 after an initial dramatic response. The resulting profile identified an activating mutation at codon 121 in the downstream kinase MEK1 that was absent in the corresponding pretreatment tumor. The MEK1(C121S) mutation was shown to increase kinase activity and confer robust resistance to both RAF and MEK inhibition in vitro. Thus, MEK1(C121S) or functionally similar mutations are predicted to confer resistance to combined MEK/RAF inhibition. These results provide an instructive framework for assessing mechanisms of acquired resistance to kinase inhibition and illustrate the use of emerging technologies in a manner that may accelerate personalized cancer medicine.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Druker BJ. Inhibition of the Bcr-Abl tyrosine kinase as a therapeutic strategy for CML. Oncogene. 2002;21:8541–8546. - PubMed
-
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. - PubMed
-
- Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. - PubMed
-
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. - PubMed
-
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

