IL-2 induces a WAVE2-dependent pathway for actin reorganization that enables WASp-independent human NK cell function
- PMID: 21383498
- PMCID: PMC3069781
- DOI: 10.1172/JCI44862
IL-2 induces a WAVE2-dependent pathway for actin reorganization that enables WASp-independent human NK cell function
Abstract
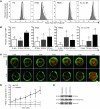
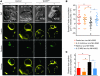
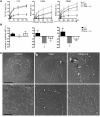
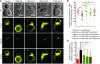
Wiskott-Aldrich syndrome (WAS) is a primary immunodeficiency associated with an increased susceptibility to herpesvirus infection and hematologic malignancy as well as a deficiency of NK cell function. It is caused by defective WAS protein (WASp). WASp facilitates filamentous actin (F-actin) branching and is required for F-actin accumulation at the NK cell immunological synapse and NK cell cytotoxicity ex vivo. Importantly, the function of WASp-deficient NK cells can be restored in vitro after exposure to IL-2, but the mechanisms underlying this remain unknown. Using a WASp inhibitor as well as cells from patients with WAS, we have defined a direct effect of IL-2 signaling upon F-actin that is independent of WASp function. We found that IL-2 treatment of a patient with WAS enhanced the cytotoxicity of their NK cells and the F-actin content at the immunological synapses formed by their NK cells. IL-2 stimulation of NK cells in vitro activated the WASp homolog WAVE2, which was required for inducing WASp-independent NK cell function, but not for baseline activity. Thus, WAVE2 and WASp define parallel pathways to F-actin reorganization and function in human NK cells; although WAVE2 was not required for NK cell innate function, it was accessible through adaptive immunity via IL-2. These results demonstrate how overlapping cytoskeletal activities can utilize immunologically distinct pathways to achieve synonymous immune function.
Figures



References
-
- Cannon JL, Burkhardt JK. Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production. J Immunol. 2004;173(3):1658–1662. - PubMed
-
- Kenney D, Cairns L, Remold-O’Donnell E, Peterson J, Rosen FS, Parkman R. Morphological abnormalities in the lymphocytes of patients with the Wiskott-Aldrich syndrome. Blood. 1986;68(6):1329–1332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

