Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFβ signaling
- PMID: 21383698
- PMCID: PMC3136543
- DOI: 10.1038/onc.2011.44
Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFβ signaling
Abstract
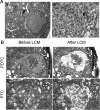
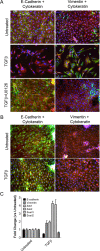
Mice with thyroid-specific expression of oncogenic BRAF (Tg-Braf) develop papillary thyroid cancers (PTCs) that are locally invasive and have well-defined foci of poorly differentiated thyroid carcinoma (PDTC). To investigate the PTC-PDTC progression, we performed a microarray analysis using RNA from paired samples of PDTC and PTC collected from the same animals by laser capture microdissection. Analysis of eight paired samples revealed a profound deregulation of genes involved in cell adhesion and intracellular junctions, with changes consistent with an epithelial-mesenchymal transition (EMT). This was confirmed by immunohistochemistry, as vimentin expression was increased and E-cadherin lost in PDTC compared with adjacent PTC. Moreover, PDTC stained positively for phospho-Smad2, suggesting a role for transforming growth factor (TGF)β in mediating this process. Accordingly, TGFβ-induced EMT in primary cultures of thyroid cells from Tg-Braf mice, whereas wild-type thyroid cells retained their epithelial features. TGFβ-induced Smad2 phosphorylation, transcriptional activity and induction of EMT required mitogen-activated protein kinase (MAPK) pathway activation in Tg-Braf thyrocytes. Hence, tumor initiation by oncogenic BRAF renders thyroid cells susceptible to TGFβ-induced EMT, through a MAPK-dependent process.
Figures




References
-
- Ball DW, Jin N, Rosen DM, Dackiw A, Sidransky D, Xing M, et al. J Clin Endocrinol Metab. 2007;92:4712–4718. - PubMed
-
- Benjamini Y, Hochberg Y. Journal of the Royal Statistical Society B. 1995;57:289–300.
-
- Bravo SB, Pampin S, Cameselle-Teijeiro J, Carneiro C, Dominguez F, Barreiro F, et al. Oncogene. 2003;22:7819–7830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

