Supraselective intra-arterial chemotherapy: evaluation of treatment-related complications in advanced retinoblastoma
- PMID: 21383945
- PMCID: PMC3045066
- DOI: 10.2147/OPTH.S12665
Supraselective intra-arterial chemotherapy: evaluation of treatment-related complications in advanced retinoblastoma
Abstract
Purpose: The purpose of this study is to report the complication profile and safety evaluation of supraselective intra-arterial melphalan chemotherapy in children undergoing treatment with advanced retinoblastoma.
Methods: Twelve eyes of 10 children with advanced retinoblastoma (Reese-Ellsworth Group Vb or International Classification Group D) were treated with supraselective intra-ophthalmic artery infusion of melphalan. Eleven eyes of nine children had previously failed traditional management with systemic chemotherapy and laser ablation and underwent intra-ophthalmic artery infusion of melphalan as an alternative to enucleation. Serial ophthalmic examinations, retinal photography, and ultrasonographic imaging were used to evaluate treatment regime.
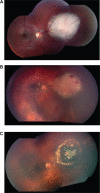
Results: Ophthalmic artery cannulation was successfully performed in 12 eyes of 10 patients (total 16 times). Striking regression of tumor, subretinal and vitreous seeds were seen early in each case. No severe systemic side effects occurred. Grade III neutropenia was seen in one patient. No transfusions were required. Three patients developed a vitreous hemorrhage obscuring tumor visualization. One patient developed periocular edema associated with inferior rectus muscle inflammation per orbital MRI. This same patient had scattered intraretinal hemorrhages and peripapillary cotton wool spots consistent with a Purtscher's-like retinopathy that resolved spontaneously. At the 6-month follow-up examination, nine eyes had no evidence of tumor progression, whereas three eyes were enucleated for tumor progression. In each enucleated case, viable tumor was identified on histopathologic examination.
Conclusions: Ophthalmic intra-arterial infusion with melphalan is an excellent globe-conserving treatment option in advanced retinoblastoma cases with minimal systemic side effects. Local toxicities include microemboli to the retina and choroid (1/12, 8%), vitreous hemorrhage (3/12, 25%), and myositis (1/12, 8%). Enucleation remained a definitive treatment for tumor progression in 3 of 12 eyes in this small case series with limited follow-up. Further studies are necessary to establish the role of supraselective intra-arterial melphalan chemotherapy for children with retinoblastoma.
Keywords: intra-arterial chemotherapy; melphalan; retinoblastoma.
Figures


References
-
- Abramson DH. Retinoblastoma incidence in the United States. Arch Ophthalmol. 1990;108(11):1514. - PubMed
-
- Tamboli A, Podgor MJ, Horm JW. The incidence of retinoblastoma in the United States: 1974 through 1985. Arch Ophthalmol. 1990;108:128–132. - PubMed
-
- Cassady JR, Sagerman RH, Tretter P, Ellsworth RM. Radiation therapy in retinoblastoma. An analysis of 230 cases. Radiology. 1969;93:405–409. - PubMed
-
- Desjardins L, Haye C, Schlienger P, Laurent M, Zucker JM, Bouguila H. Second non-ocular tumours in survivors of bilateral retinoblastoma. A 30-year follow-up. Ophthalmic Paediatr Genet. 1991;12(3):145–148. - PubMed
-
- Marees T, Moll AC, Imhof SM, de Boer MR, Ringens PJ, van Leeuwen FE. Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst. 2008;100(24):1743–1745. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

