From glycosylation disorders to dolichol biosynthesis defects: a new class of metabolic diseases
- PMID: 21384228
- PMCID: PMC3137772
- DOI: 10.1007/s10545-011-9301-0
From glycosylation disorders to dolichol biosynthesis defects: a new class of metabolic diseases
Abstract
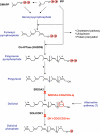
Polyisoprenoid alcohols are membrane lipids that are present in every cell, conserved from archaea to higher eukaryotes. The most common form, alpha-saturated polyprenol or dolichol is present in all tissues and most organelle membranes of eukaryotic cells. Dolichol has a well defined role as a lipid carrier for the glycan precursor in the early stages of N-linked protein glycosylation, which is assembled in the endoplasmic reticulum of all eukaryotic cells. Other glycosylation processes including C- and O-mannosylation, GPI-anchor biosynthesis and O-glucosylation also depend on dolichol biosynthesis via the availability of dolichol-P-mannose and dolichol-P-glucose in the ER. The ubiquity of dolichol in cellular compartments that are not involved in glycosylation raises the possibility of additional functions independent of these protein post-translational modifications. The molecular basis of several steps involved in the synthesis and the recycling of dolichol and its derivatives is still unknown, which hampers further research into this direction. In this review, we summarize the current knowledge on structural and functional aspects of dolichol metabolites. We will describe the metabolic disorders with a defect in known steps of dolichol biosynthesis and recycling in human and discuss their pathogenic mechanisms. Exploration of the developmental, cellular and biochemical defects associated with these disorders will provide a better understanding of the functions of this lipid class in human.
Figures

References
-
- Carson DD, Earles BJ, et al. (1981) Enhancement of protein glycosylation in tissue slices by dolichylphosphate. J Biol Chem 256(22):11552–11557 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

