Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis
- PMID: 21385452
- PMCID: PMC3219186
- DOI: 10.1186/bcr2841
Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis
Abstract
Introduction: Detection of serum biomarkers for early diagnosis of breast cancer remains an important goal. Changes in the structure of O-linked glycans occur in all breast cancers resulting in the expression of glycoproteins that are antigenically distinct. Indeed, the serum assay widely used for monitoring disease progression in breast cancer (CA15.3), detects a glycoprotein (MUC1), but elevated levels of the antigen cannot be detected in early stage patients. However, since the immune system acts to amplify the antigenic signal, antibodies can be detected in sera long before the antigen. We have exploited the change in O-glycosylation to measure autoantibody responses to cancer-associated glycoforms of MUC1 in sera from early stage breast cancer patients.
Methods: We used a microarray platform of 60mer MUC1 glycopeptides, to confirm the presence of autoantibodies to cancer associated glycoforms of MUC1 in a proportion of early breast cancer patients (54/198). Five positive sera were selected for detailed definition of the reactive epitopes using on chip glycosylation technology and a panel of glycopeptides based on a single MUC1 tandem repeat carrying specific glycans at specific sites. Based on these results, larger amounts of an extended repertoire of defined MUC1 glycopeptides were synthesised, printed on microarrays, and screened with sera from a large cohort of breast cancer patients (n = 395), patients with benign breast disease (n = 108) and healthy controls (n = 99). All sera were collected in the 1970s and 1980s and complete clinical follow-up of breast cancer patients is available.
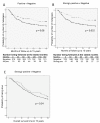
Results: The presence and level of autoantibodies was significantly higher in the sera from cancer patients compared with the controls, and a highly significant correlation with age was observed. High levels of a subset of autoantibodies to the core3MUC1 (GlcNAcβ1-3GalNAc-MUC1) and STnMUC1 (NeuAcα2,6GalNAc-MUC1) glycoforms were significantly associated with reduced incidence and increased time to metastasis.
Conclusions: Autoantibodies to specific cancer associated glycoforms of MUC1 are found more frequently and at higher levels in early stage breast cancer patients than in women with benign breast disease or healthy women. Association of strong antibody response with reduced rate and delay in metastases suggests that autoantibodies can affect disease progression.
Figures




References
-
- Desmetz C, Bascoul-Mollevi C, Rochaix P, Lamy PJ, Kramar A, Rouanet P, Maudelonde T, Mangé A, Solassol J. Identification of a new panel of serum autoantibodies associated with the presence of in situ carcinoma of the breast in younger women. Clin Cancer Res. 2009;15:4733–4741. doi: 10.1158/1078-0432.CCR-08-3307. - DOI - PubMed
-
- Lumachi F, Basso SM, Brandes AA, Pagano D, Ermani M. Relationship between tumor markers CEA and CA 15-3, TNM staging, estrogen receptor rate and MIB-1 index in patients with pT1-2 breast cancer. Anticancer Res. 2004;24:3221–3224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

