Shipping blood to a central laboratory in multicenter clinical trials: effect of ambient temperature on specimen temperature, and effects of temperature on mononuclear cell yield, viability and immunologic function
- PMID: 21385453
- PMCID: PMC3063218
- DOI: 10.1186/1479-5876-9-26
Shipping blood to a central laboratory in multicenter clinical trials: effect of ambient temperature on specimen temperature, and effects of temperature on mononuclear cell yield, viability and immunologic function
Abstract
Background: Clinical trials of immunologic therapies provide opportunities to study the cellular and molecular effects of those therapies and may permit identification of biomarkers of response. When the trials are performed at multiple centers, transport and storage of clinical specimens become important variables that may affect lymphocyte viability and function in blood and tissue specimens. The effect of temperature during storage and shipment of peripheral blood on subsequent processing, recovery, and function of lymphocytes is understudied and represents the focus of this study.
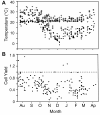
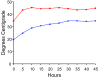
Methods: Peripheral blood samples (n = 285) from patients enrolled in 2 clinical trials of a melanoma vaccine were shipped from clinical centers 250 or 1100 miles to a central laboratory at the sponsoring institution. The yield of peripheral blood mononuclear cells (PBMC) collected before and after cryostorage was correlated with temperatures encountered during shipment. Also, to simulate shipping of whole blood, heparinized blood from healthy donors was collected and stored at 15 °C, 22 °C, 30 °C, or 40 °C, for varied intervals before isolation of PBMC. Specimen integrity was assessed by measures of yield, recovery, viability, and function of isolated lymphocytes. Several packaging systems were also evaluated during simulated shipping for the ability to maintain the internal temperature in adverse temperatures over time.
Results: Blood specimen containers experienced temperatures during shipment ranging from -1 to 35 °C. Exposure to temperatures above room temperature (22 °C) resulted in greater yields of PBMC. Reduced cell recovery following cryo-preservation as well as decreased viability and immune function were observed in specimens exposed to 15 °C or 40 °C for greater than 8 hours when compared to storage at 22 °C. There was a trend toward improved preservation of blood specimen integrity stored at 30 °C prior to processing for all time points tested. Internal temperatures of blood shipping containers were maintained longer in an acceptable range when warm packs were included.
Conclusions: Blood packages shipped overnight by commercial carrier may encounter extreme seasonal temperatures. Therefore, considerations in the design of shipping containers should include protecting against extreme ambient temperature deviations and maintaining specimen temperature above 22 °C or preferably near 30 °C.
Figures

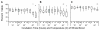

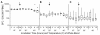

References
-
- Vaught JB, Caboux E, Hainaut P. International efforts to develop biospecimen best practices. Cancer Epidemiology Biomarkers & Prevention. 2010;19:912–915. - PubMed
-
- Hallmans G, Vaught JB. In: Methods in Biobanking. Dillner J, editor. Springer Science+Business Media, LLC; 2011. Best Practices for Establishing a Biobank; pp. 241–260. full_text. - PubMed
-
- Leyland-Jones BR, Ambrosone CB, Bartlett J, Ellis MJC, Enos RA, Raji A, Pins MR, Zujewski JA, Hewitt SM, Forbes JF, Abramovitz M, Braga S, Cardoso F, Harbeck N, Denkert C, Jewell SD. Recommendations for collection and handling of specimens from group breast cancer clinical trials. J Clin Oncol. 2008;26:5638–5644. doi: 10.1200/JCO.2007.15.1712. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

