Controlling and coordinating development in vector-transmitted parasites
- PMID: 21385707
- PMCID: PMC3834321
- DOI: 10.1126/science.1198077
Controlling and coordinating development in vector-transmitted parasites
Erratum in
- Science. 2011 Apr 22;332(6028):421
Abstract
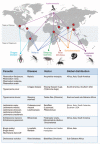
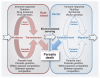
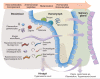
Vector-borne parasites cause major human diseases of the developing world, including malaria, human African trypanosomiasis, Chagas disease, leishmaniasis, filariasis, and schistosomiasis. Although the life cycles of these parasites were defined over 100 years ago, the strategies they use to optimize their successful transmission are only now being understood in molecular terms. Parasites are now known to monitor their environment in both their host and vector and in response to other parasites. This allows them to adapt their developmental cycles and to counteract any unfavorable conditions they encounter. Here, I review the interactions that parasites engage in with their hosts and vectors to maximize their survival and spread.
Figures
References
-
- Davies SJ, et al. Science. 2001;294:1358. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

