Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques
- PMID: 21385847
- PMCID: PMC3100690
- DOI: 10.1182/blood-2010-10-311456
Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques
Abstract
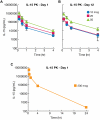
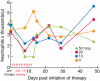
IL-15 uses the heterotrimeric receptor IL-2/IL-15Rβ and the γ chain shared with IL-2 and the cytokine-specific IL-15Rα. Although IL-15 shares actions with IL-2 that include activation of natural killer (NK) and CD8 T cells, IL-15 is not associated with capillary leak syndrome, activation-induced cell death, or with a major effect on the number of functional regulatory T cells. To prepare for human trials to determine whether IL-15 is superior to IL-2 in cancer therapy, recombinant human IL-15 (rhIL-15) was produced under current good manufacturing practices. A safety study in rhesus macaques was performed in 4 groups of 6 animals each that received vehicle diluent control or rhIL-15 at 10, 20, or 50 μg/kg/d IV for 12 days. The major toxicity was grade 3/4 transient neutropenia. Bone marrow examinations demonstrated increased marrow cellularity, including cells of the neutrophil series. Furthermore, neutrophils were observed in sinusoids of enlarged livers and spleens, suggesting that IL-15 mediated neutrophil redistribution from the circulation to tissues. The observation that IL-15 administration was associated with increased numbers of circulating NK and CD8 central and effector-memory T cells, in conjunction with efficacy studies in murine tumor models, supports the use of multiple daily infusions of rhIL-15 in patients with metastatic malignancies.
Figures


References
-
- Wadler S, Schwartz EL. Antineoplastic activity of the combination of interferon and cytotoxic agents against experimental and human malignancies: a review. Cancer Res. 1990;50(12):3473–3486. - PubMed
-
- Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA. 1994;271(12):907–913. - PubMed
-
- Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6(11):1071–1072. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

