Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia
- PMID: 21385853
- PMCID: PMC3099570
- DOI: 10.1182/blood-2010-10-310425
Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia
Abstract
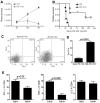
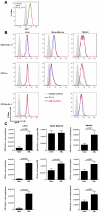
Tumor-associated immune suppression can lead to defective T cell-mediated antitumor immunity. Here, we identified a unique phenotype of exhausted T cells in mice with advanced acute myelogenous leukemia (AML). This phenotype is characterized by the coexpression of Tim-3 and PD-1 on CD8(+) T cells in the liver, the major first site of AML metastases. PD-1 and Tim-3 coexpression increased during AML progression. PD-1(+)Tim-3(+) CD8(+) T cells were deficient in their ability to produce IFN-γ, TNF-α, and IL-2 in response to PD-1 ligand (PDL1) and Tim-3 ligand (galectin-9) expressing AML cells. PD-1 knockout (KO), which were partially resistant to AML challenge, up-regulated Tim-3 during AML progression and such Tim-3(+)PD-1- KO CD8(+) T cells had reduced cytokine production. Galectin-9 KO mice were more resistant to AML, which was associated with reduced T-regulatory cell accumulation and a modest induction of PD-1 and Tim-3 expression on CD8(+) T cells. Whereas blocking the PD-1/PDL1 or Tim-3/galectin-9 pathway alone was insufficient to rescue mice from AML lethality, an additive effect was seen in reducing-albeit not eliminating-both tumor burden and lethality when both pathways were blocked. Therefore, combined PD-1/PDL1 and Tim-3/galectin-9 blockade may be beneficial in preventing CD8(+) T-cell exhaustion in patients with hematologic malignancies such as advanced AML.
Figures




References
-
- Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

