A developmental taxonomy of glioblastoma defined and maintained by MicroRNAs
- PMID: 21385897
- PMCID: PMC3085663
- DOI: 10.1158/0008-5472.CAN-10-4117
A developmental taxonomy of glioblastoma defined and maintained by MicroRNAs
Abstract
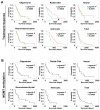
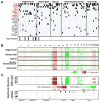
mRNA expression profiling has suggested the existence of multiple glioblastoma subclasses, but their number and characteristics vary among studies and the etiology underlying their development is unclear. In this study, we analyzed 261 microRNA expression profiles from The Cancer Genome Atlas (TCGA), identifying five clinically and genetically distinct subclasses of glioblastoma that each related to a different neural precursor cell type. These microRNA-based glioblastoma subclasses displayed microRNA and mRNA expression signatures resembling those of radial glia, oligoneuronal precursors, neuronal precursors, neuroepithelial/neural crest precursors, or astrocyte precursors. Each subclass was determined to be genetically distinct, based on the significant differences they displayed in terms of patient race, age, treatment response, and survival. We also identified several microRNAs as potent regulators of subclass-specific gene expression networks in glioblastoma. Foremost among these is miR-9, which suppresses mesenchymal differentiation in glioblastoma by downregulating expression of JAK kinases and inhibiting activation of STAT3. Our findings suggest that microRNAs are important determinants of glioblastoma subclasses through their ability to regulate developmental growth and differentiation programs in several transformed neural precursor cell types. Taken together, our results define developmental microRNA expression signatures that both characterize and contribute to the phenotypic diversity of glioblastoma subclasses, thereby providing an expanded framework for understanding the pathogenesis of glioblastoma in a human neurodevelopmental context.
Figures




References
-
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–23. - PubMed
-
- Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–31. - PubMed
-
- Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

