Androgenic influence on serotonergic activation of the HPA stress axis
- PMID: 21385938
- PMCID: PMC3075941
- DOI: 10.1210/en.2010-0964
Androgenic influence on serotonergic activation of the HPA stress axis
Abstract
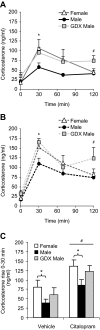
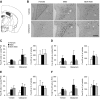
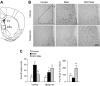
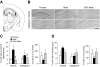
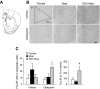
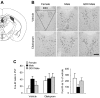
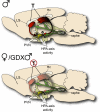
The higher incidence of stress-mediated affective disorders in women may be a function of gonadal hormone influence on complex interactions between serotonin and neural circuits that mediate the hypothalamic-pituitary-adrenal (HPA) stress axis. The paraventricular nucleus of the hypothalamus (PVN) receives serotonergic innervation, and selective serotonin reuptake inhibitors such as citalopram activate the HPA axis independent of stress. We have previously demonstrated that the magnitude of this serotonergic activation was greater in females and was attenuated by testosterone administration; however, the potential central sites of action where androgens reduce these serotonergic effects have not been determined. Therefore, we examined a time course of corticosterone production and used central c-Fos protein levels to assay neuronal activation in stress-related brain regions in female, male, and gonadectomized male mice after an acute citalopram injection (15 mg/kg). In the hippocampus, c-Fos-immunoreactivity was greater in males than in females or gonadectomized males. This same pattern emerged in the lateral septum after vehicle and gonadectomy reversed the effect of citalopram. These regions are important for inhibitory influences on the PVN, and accordingly, hippocampal c-Fos levels were negatively correlated with corticosterone production. No sex differences in c-Fos were detected in the PVN, cingulate cortex, or paraventricular thalamus in response to vehicle or citalopram. These data support brain region-specific regulation of the HPA axis where sex differences may be mediated partly through androgen enhancement of signaling in inhibitory regions.
Figures
References
-
- Kendler KS, Karkowski LM, Prescott CA. 1999. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841 - PubMed
-
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602 - PubMed
-
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. 1999. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160:1–12 - PubMed
-
- Bale TL. 2005. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav 48:1–10 - PubMed
-
- Holden C. 2005. Sex and the suffering brain. Science 308:1574. - PubMed

