Biotechnology in the treatment of sensorineural hearing loss: foundations and future of hair cell regeneration
- PMID: 21386039
- PMCID: PMC3163053
- DOI: 10.1044/1092-4388(2011/10-0149)
Biotechnology in the treatment of sensorineural hearing loss: foundations and future of hair cell regeneration
Abstract
Purpose: To provide an overview of the methodologies involved in the field of hair cell regeneration. First, the author provides a tutorial on the biotechnological foundations of this field to assist the reader in the comprehension and interpretation of the research involved in hair cell regeneration. Next, the author presents a review of stem cell and gene therapy and provides a critical appraisal of their application to hair cell regeneration. The methodologies used in these approaches are highlighted.
Method: The author conducted a narrative review of the fields of cellular, molecular, and developmental biology, tissue engineering, and stem cell and gene therapy using the PubMed database.
Results: The use of biotechnological approaches to the treatment of hearing loss--approaches such as stem cell and gene therapy-has led to new methods of regenerating cochlear hair cells in mammals.
Conclusions: Incredible strides have been made in assembling important pieces of the puzzle that comprise hair cell regeneration. However, mammalian hair cell regeneration using stem cell and gene therapy are years--if not decades--away from being clinically feasible. If the goals of the biological approaches are met, these therapies may represent future treatments for hearing loss.
Figures

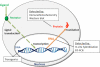



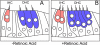



References
-
- Abello G, Alsina B. Establishment of a proneural field in the inner ear. Int J Dev Biol. 2007;51(6-7):483–493. - PubMed
-
- Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270(15):8730–8738. - PubMed
-
- Alberts B, Johnson A, Lewis J, Raff M. Molecular Biology of the Cell. 5 ed. Garland Science; New York: 2007.
-
- Alford BR, Ruben RJ. Physiological, behavioral and anatomical correlates of the development of hearing in the mouse. Ann Otol Rhinol Laryngol. 1963;72:237–247. - PubMed
-
- Alibardi L. Morphological and cellular aspects of tail and limb regeneration in lizards. A model system with implications for tissue regeneration in mammals. Adv Anat Embryol Cell Biol. 2010;207:iii, v–x, 1–109. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

