A new MRI rating scale for progressive supranuclear palsy and multiple system atrophy: validity and reliability
- PMID: 21386111
- PMCID: PMC3152869
- DOI: 10.1136/jnnp.2010.214890
A new MRI rating scale for progressive supranuclear palsy and multiple system atrophy: validity and reliability
Abstract
Aim: To evaluate a standardised MRI acquisition protocol and a new image rating scale for disease severity in patients with progressive supranuclear palsy (PSP) and multiple systems atrophy (MSA) in a large multicentre study.
Methods: The MRI protocol consisted of two-dimensional sagittal and axial T1, axial PD, and axial and coronal T2 weighted acquisitions. The 32 item ordinal scale evaluated abnormalities within the basal ganglia and posterior fossa, blind to diagnosis. Among 760 patients in the study population (PSP = 362, MSA = 398), 627 had per protocol images (PSP = 297, MSA = 330). Intra-rater (n = 60) and inter-rater (n = 555) reliability were assessed through Cohen's statistic, and scale structure through principal component analysis (PCA) (n = 441). Internal consistency and reliability were checked. Discriminant and predictive validity of extracted factors and total scores were tested for disease severity as per clinical diagnosis.
Results: Intra-rater and inter-rater reliability were acceptable for 25 (78%) of the items scored (≥ 0.41). PCA revealed four meaningful clusters of covarying parameters (factor (F) F1: brainstem and cerebellum; F2: midbrain; F3: putamen; F4: other basal ganglia) with good to excellent internal consistency (Cronbach α 0.75-0.93) and moderate to excellent reliability (intraclass coefficient: F1: 0.92; F2: 0.79; F3: 0.71; F4: 0.49). The total score significantly discriminated for disease severity or diagnosis; factorial scores differentially discriminated for disease severity according to diagnosis (PSP: F1-F2; MSA: F2-F3). The total score was significantly related to survival in PSP (p<0.0007) or MSA (p<0.0005), indicating good predictive validity.
Conclusions: The scale is suitable for use in the context of multicentre studies and can reliably and consistently measure MRI abnormalities in PSP and MSA. Clinical Trial Registration Number The study protocol was filed in the open clinical trial registry (http://www.clinicaltrials.gov) with ID No NCT00211224.
Conflict of interest statement
Figures

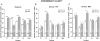
References
-
- Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet 1999;354:1771–5 - PubMed
-
- Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome): report of the NINDS-SPSP International Workshop. Neurology 1996;47:1–9 - PubMed
-
- Wenning GK, Ben SY, Magalhaes M, et al. Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain 1994;117:835–45 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
