A simple indentation device for measuring micrometer-scale tissue stiffness
- PMID: 21386443
- PMCID: PMC3392911
- DOI: 10.1088/0953-8984/22/19/194120
A simple indentation device for measuring micrometer-scale tissue stiffness
Abstract
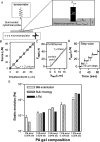
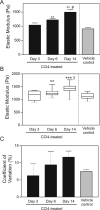
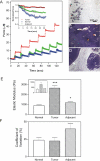
Mechanical properties of cells and extracellular matrices are critical determinants of function in contexts including oncogenic transformation, neuronal synapse formation, hepatic fibrosis and stem cell differentiation. The size and heterogeneity of biological specimens and the importance of measuring their mechanical properties under conditions that resemble their environments in vivo present a challenge for quantitative measurement. Centimeter-scale tissue samples can be measured by commercial instruments, whereas properties at the subcellular (nm) scale are accessible by atomic force microscopy, optical trapping, or magnetic bead microrheometry; however many tissues are heterogeneous on a length scale between micrometers and millimeters which is not accessible to most current instrumentation. The device described here combines two commercially available technologies, a micronewton resolution force probe and a micromanipulator for probing soft biological samples at sub-millimeter spatial resolution. Several applications of the device are described. These include the first measurement of the stiffness of an intact, isolated mouse glomerulus, quantification of the inner wall stiffness of healthy and diseased mouse aortas, and evaluation of the lateral heterogeneity in the stiffness of mouse mammary glands and rat livers with correlation of this heterogeneity with malignant or fibrotic pathology as evaluated by histology.
Figures


References
-
- Boyd NF, Martin LJ, Rommens JM, Paterson AD, Minkin S, Yaffe MJ, Stone J, Hopper JL. Mammographic density: a heritable risk factor for breast cancer. Methods Mol. Biol. 2009;472:343–60. - PubMed
-
- Cheng L, Xia X, Yu W, Scriven LE, Gerberich WW. Flat-punch indentation of viscoelastic material. J. Polym. Sci. B. 1999;38:10–22.
-
- Deuschle J, Enders S, Arzt E. Surface detection in nanoindentation of soft polymers. J. Mater. Res. 2007;22:3107–19.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
