FOLFOX6 and bevacizumab in non-optimally resectable liver metastases from colorectal cancer
- PMID: 21386839
- PMCID: PMC3068493
- DOI: 10.1038/bjc.2011.43
FOLFOX6 and bevacizumab in non-optimally resectable liver metastases from colorectal cancer
Abstract
Background: In patients with colorectal liver metastases (CLM) R0 resection significantly improves overall survival (OS).
Methods: In this report, we present the results of a phase II trial of FOLFOX6+bevacizumab in patients with non-optimally resectable CLM. Patients received six cycles of FOLFOX6+ five of bevacizumab. Patients not achieving resectability received six additional cycles of each. A PET-CT was performed at baseline and again within 1 month after initiating treatment.
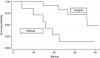
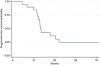
Results: From September 2005 to July 2009, 21 patients were enrolled (Male/Female: 15/6; median age: 65 years). An objective response (OR) was documented in 12 cases (57.1%; complete responses (CRs): 3, partial response (PR): 9); one patient died from toxicity before surgery. Thirteen patients underwent radical surgery (61.9%). Three (23%) had a pathological CR (pCR). Six patients (46.1%) experienced minor postsurgical complications. After a median 38.8-month follow-up, the median OS was 22.5 months. Patients achieving at least 1 unit reduction in Standard uptake value (SUV)max on PET-CT had longer progression-free survival (PFS) (median PFS: 22 vs 14 months, P=0.001).
Conclusions: FOLFOX6+bevacizumab does not increase postsurgical complications, yields high rates of resectability and pCR. Early changes in PET-CT seem to be predictive of longer PFS.
Figures
References
-
- Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O, Levi F, Bismuth H (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 240: 644–657 - PMC - PubMed
-
- Benoist S, Brouquet A, Penna C, Julié C, El Hajjam M, Chagnon S, Mitry E, Rougier P, Nordlinger B (2006) Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol 4: 3939–3945 - PubMed
-
- Blazer 3rd DG, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, Zorzi D, Ribero D, Ellis LM, Glover KY, Wolff RA, Curley SA, Abdalla EK, Vauthey JN (2008) Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 26: 5344–5351 - PubMed
-
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27: 663–671 - PubMed
-
- Bouganim N (2009) Perioperative chemotherapy with bevacizumab (BV) for liver metastases (LM) in colorectal cancer (CRC): McGill University pilot study. 2009 ASCO Annual Meeting, Orlando, e15027
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials