Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo
- PMID: 21386905
- PMCID: PMC3046136
- DOI: 10.1371/journal.pone.0016722
Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo
Abstract
Background: Age-related macular degeneration (AMD) is the leading cause of legal blindness in the elderly population. Debris (termed drusen) below the retinal pigment epithelium (RPE) have been recognized as a risk factor for dry AMD and its progression to wet AMD, which is characterized by choroidal neovascularization (CNV). The underlying mechanism of how drusen might elicit CNV remains undefined. Cigarette smoking, oxidative damage to the RPE and inflammation are postulated to be involved in the pathophysiology of the disease. To better understand the cellular mechanism(s) linking oxidative stress and inflammation to AMD, we examined the expression of pro-inflammatory monocyte chemoattractant protein-1 (MCP-1), pro-angiogenic vascular endothelial growth factor (VEGF) and anti-angiogenic pigment epithelial derived factor (PEDF) in RPE from smoker patients with AMD. We also evaluated the effects of hydroquinone (HQ), a major pro-oxidant in cigarette smoke on MCP-1, VEGF and PEDF expression in cultured ARPE-19 cells and RPE/choroids from C57BL/6 mice.
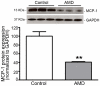
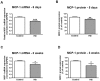
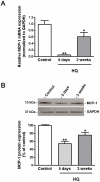
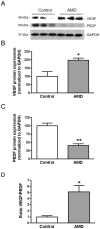
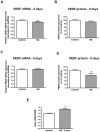
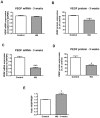
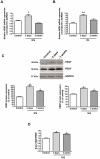
Principal findings: MCP-1, VEGF and PEDF expression was examined by real-time PCR, Western blot, and ELISA. Low levels of MCP-1 protein were detected in RPE from AMD smoker patients relative to controls. Both MCP-1 mRNA and protein were downregulated in ARPE-19 cells and RPE/choroids from C57BL/6 mice after 5 days and 3 weeks of exposure to HQ-induced oxidative injury. VEGF protein expression was increased and PEDF protein expression was decreased in RPE from smoker patients with AMD versus controls resulting in increased VEGF/PEDF ratio. Treatment with HQ for 5 days and 3 weeks increased the VEGF/PEDF ratio in vitro and in vivo.
Conclusion: We propose that impaired RPE-derived MCP-1-mediated scavenging macrophages recruitment and phagocytosis might lead to incomplete clearance of proinflammatory debris and infiltration of proangiogenic macrophages which along with increased VEGF/PEDF ratio favoring angiogenesis might promote drusen accumulation and progression to CNV in smoker patients with dry AMD.
Conflict of interest statement
Figures
References
-
- Augood CA, Vingerling JR, de Jong PT, Chakravarthy U, Seland J, et al. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE). Arch Ophthalmol. 2006;124:529–535. - PubMed
-
- Evans JR. Risk factors for age-related macular degeneration. Prog Retin Eye Res. 2001;20:227–253. - PubMed
-
- Javitt JC, Zhou Z, Maguire MG, Fine SL, Willke RJ. Incidence of exudative age-related macular degeneration among elderly Americans. Ophthalmology. 2003;110:1534–1539. - PubMed
-
- Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–495. - PubMed
-
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

